
23 March 2020
Amy S Van Wey Lovatt
Via FYI.org.nz
Email:
[FYI request #12431 email]
Dear Ms Van Wey Lovatt
Official Information Act (1982) Request
I write in response to your Official Information Act request, received by us on 11 March 2020. You
requested the following information.
Request 1:
•
I am writing to request a copy of the CMDHB best practice protocol for the archiving, storage,
or biobanking of human tissue for diagnostic purposes, whether they are formalin fixed,
paraffin embedded or pathology slides, and the standards, legislation or scientific evidence
which provides the basis for the CMDHB protocol.
Request 2:
•
Is the CMDHB best practice protocol for the archiving, storage, or biobanking of human
tissue for diagnostic purposes, whether they are formalin fixed, paraffin embedded or
pathology slides, is a standard which is universally adopted by all NZ DHB's and medical
laboratories, or does each DHB or medical laboratory determine their own best practice?
Request 3:
•
Please explain the potential hazards of leaving formalin fixed, paraffin embedded or
pathology slides in an unsecure, non-temperature regulated environment (say a on an
employee's desk outside of the laboratory) for 2 months.
Request 4:
•
Please provide me with the name of the independent agency or agencies which provides
oversight for medical laboratories in NZ.
Thank you for your assistance in this matter.
Please note, this request is for my information and is not a reflection upon the practices at CMDHB, but
to ascertain if there is a universal standard within NZ. I will be making this same request to every DHB
in NZ.
As context, Counties Manukau Health (CM Health) provides health and support services to people
living in the Counties Manukau region (approx. 569,400 people). Our services are delivered via hospital,
outpatient/ ambulatory and community-based models of care. We provide regional and supra-regional
specialist services (for orthopaedics, plastics, burns and spinal services). There are also several
Counties Manukau District Health Board
Private Bag 93311, Otahuhu, Auckland
T: 09 276 0000 | cmdhb.org.nz
specialist services provided for our community via other metro Auckland DHBs, including tertiary
surgical/ medical services, some mental health and addiction services.
Our responses to each of your questions are below.
Request 1:
•
I am writing to request a copy of the CMDHB best practice protocol for the archiving, storage,
or biobanking of human tissue for diagnostic purposes, whether they are formalin fixed,
paraffin embedded or pathology slides, and the standards, legislation or scientific evidence
which provides the basis for the CMDHB protocol.
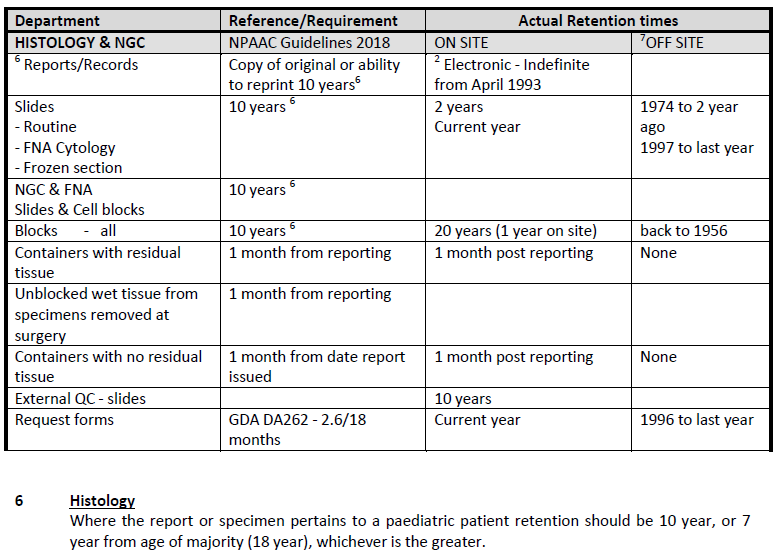
Retention of specimens/ blocks/ slides after diagnostic testing by CM Health Histology Laboratory is in
accordance with the National Pathology Accreditation Advisory Council (NPAAC) Seventh Edition 2018
(attached). That publication defines the minimum retention times, and is used in Laboratories
throughout New Zealand and Australia.
CM Health Laboratory: documented retention requirements:
The CM Health Laboratory onsite storage is secure and within the Laboratory facilities. Off-site storage
is at a secure facility.
Page 2

Request 2:
•
Is the CMDHB best practice protocol for the archiving, storage, or biobanking of human
tissue for diagnostic purposes, whether they are formalin fixed, paraffin embedded or
pathology slides, is a standard which is universally adopted by all NZ DHB's and medical
laboratories, or does each DHB or medical laboratory determine their own best practice?
The NPAAC requirements for the retention of diagnostic material noted above are used throughout
New Zealand and Australia.
Request 3:
•
Please explain the potential hazards of leaving formalin fixed, paraffin embedded or
pathology slides in an unsecure, non-temperature regulated environment (say a on an
employee's desk outside of the laboratory) for 2 months.
At the CM Health Laboratory, no employee in Histopathology has a work-desk outside of the secure
purpose-built Laboratory facility. Paraffin blocks and slides are stable, and do not pose a hazard.
Request 4:
•
Please provide me with the name of the independent agency or agencies which provides
oversight for medical laboratories in NZ.
International Accreditation New Zealand (IANZ) assesses all NZ Medical Laboratories against the
International Standard:
Medical laboratories – Requirements for quality and competence, ISO
15189:2012.
I trust this information satisfactorily answers your query. If you are not satisfied with this response you
are entitled to seek a review of the response by the Ombudsman under section 28(3) of the Official
Information Act.
Please note that this response or an edited version of this may be published on the Counties Manukau
DHB website.
Yours sincerely,
Fepulea’i Margie Apa
Chief Executive Officer
Counties Manukau Health
Page 3


