
5. When filling out the application form:
* Please read all questions and instructions carefully and refer to the
User Guide throughout.
* Answer all questions as completely and as clearly as possible. This will help the AEC understand your project and determine whether it can be
approved. If the information you provide is incomplete or not clear, your approval might be delayed.
* Please check spelling, grammar and check all sections have been completed.
* Ensure that all relevant appendices (e.g. monitoring sheets) have been attached.
*
To prevent any loss, please save your work regularly by clicking on the "save" icon located on the top left corner of this form.
Notes:
* If your response to a question exceeds 4000 characters (including spaces), please (a) write "Refer to attachment" as a response to the
question and (b) upload a word document of your response in ‘Section G: Attachments’.
* Applicants for whom English is a second language are strongly advised to have their application reviewed by someone whose first language is
English.
6. Submitting the application
Applications need to reach the Research Office by 5.00 pm on the closing date advertised on the AEC web page. After you have clicked the
‘submit’ icon, the application automatically gets routed to the Head of Department. Please allow time for the HOD to view and sign-off before
the closing time.
Applications will be pre-screened. This procedure allows the identification of any issues in the application that may prevent or delay its approval
by the committee, and will facilitate the approval process. We therefore encourage the early submission of applications.
If you have not received a letter of receipt after 3 days from submitting your application, please contact the Animal Ethics Administrator
Assistance with questions within the application form can be emailed to the Animal Ethics Administrator or by phone on 09 923 6356 (DDI) or
ext: 86356 (internal).





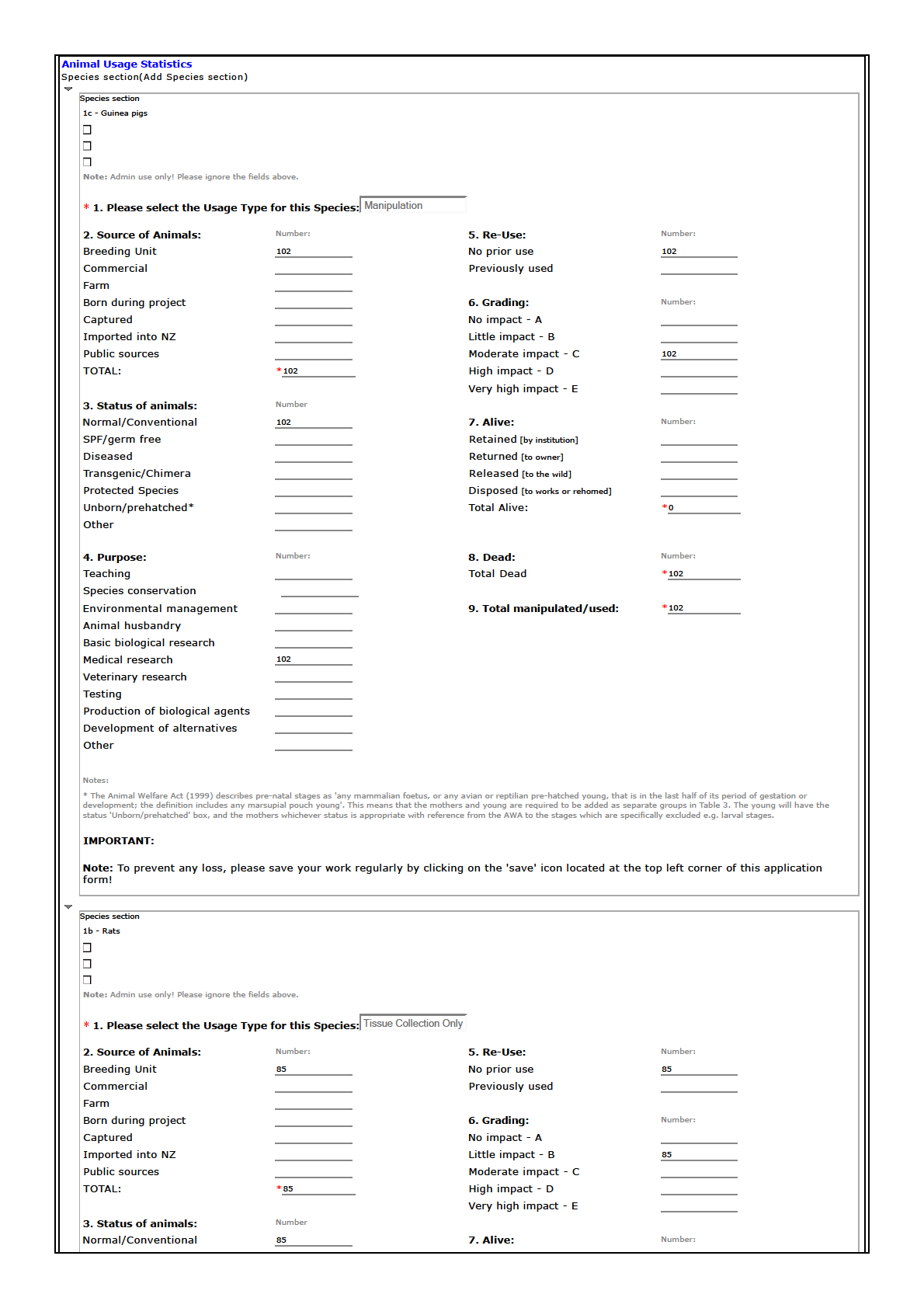

status 'Unborn/prehatched' box, and the mothers whichever status is appropriate with reference from the AWA to the stages which are specifically excluded e.g. larval stages.
IMPORTANT:
Note: To prevent any loss, please save your work regularly by clicking on the 'save' icon located at the top left corner of this application
form!

[sections 9(2)(b)(ii), 9(2)(i)]

[sections 9(2)(b)(ii), 9(2)(i)]


[sections 9(2)(b)(ii), 9(2)(i)]
We are very interested in replacement approaches
(especially for Study 1 and 2) and have assessed,
through a search of the literature (PubMed and
Medline), the possibility of using non-sentient or less
sentient (zebrafish) animals and in silico or immortal
cell line approaches for this work. Zebrafish are used
substantially to screen for ototoxic drugs (eg Ou et al.,
Drug Discov Today, 15(7-8): 265–271.
doi:10.1016/j.drudis.2010.01.001) but we have not yet
assessed whether these would be suitable as
alternatives to study otoprotective mechanisms and it is
often required to repeat these studies in mammalian
species as a proof-of-principle. There are several
immortal cochlear sensory hair cell lines (Rivolta and
Holley, 2002 J Neurobiol. 53:306-18), which have been
used to look at ototoxicity metabolic mechanisms. But
these lack the integrity of the sensory epithelium
necessary to look at the interaction of supporting and
sensory tissues, and needed for this study (the
supporting cells are considered to be involved in
organising sensory cell death). Furthermore, some of
the aminoglycoside ototoxic pathways in immortal
cochlear sensory hair cell lines are different to those
seen in vivo (Chen et al., 2012, Hearing Research
284:33-41). However, we have developed systems for
partial replacement and are using organotypic cultures
of the inner ear where possible, such as in the first two
studies described in this proposal. These are taken from
neonatal (P3-P6 mice) and do not involve any
experimental manipulation of the animal. The studies of
cochlear implantation (Study 3) require in vivo
experiments in order to assess the natural immune
response and formation of the fibrosis as it occurs in
human surgical implantation. It is not possible to use
cell lines for these particular research questions as these
do not mimic the complex relationship between the
different sensory, neural and secretory tissues involved
in the cochlear response to injury. However, we are
investigating ways to model the local changes in the
inner ear following surgery (ie those not involving a
systemic response or are confined to signal transduction
pathways expressed in cell lines) to evaluate the impact
of treatments using cell culture or in vitro systems in a
similar way to previous studies (eg Bas et al., 2015,
Frontiers of Cellular Neuroscience doi:
10.3389/fncel.2015.00303, and our previous studies
Vlajkovic et al. (1998) Hear Res.117:71-80). The
technology for developing inner ear organoids (Koehler
et al., Nature Biotechnology 35, 518–520 (2017)
doi:10.1038/nbt.3899), may eventually allow study of
localised tissue responses and mechanisms of injury.

Feedback
Please help us to improve this system by providing feedback on your experience with creating this eForm application: include all your positive
and negative experiences as well as what improvements you would like to see in using this application.
*Is this Application now complete and ready for submission?
No
Please change the response above to "Yes" once this application is complete and prior to ticking the 'Complete' checkbox (located at the top
right corner of the application). This is to hide the majority of the instruction text on the form.
Appendix 1
EForm Name: AE and Bio-Safety Form v4
Page:
Section G: Attachments
Section:
Please list all attachments appended in support of this application:
Question:
File Name:
AEC 1986 Supplementary details on the design and methods.docx
[sections 9(2)(b)(ii), 9(2)(i)]
Supplementary details on the design and methods.
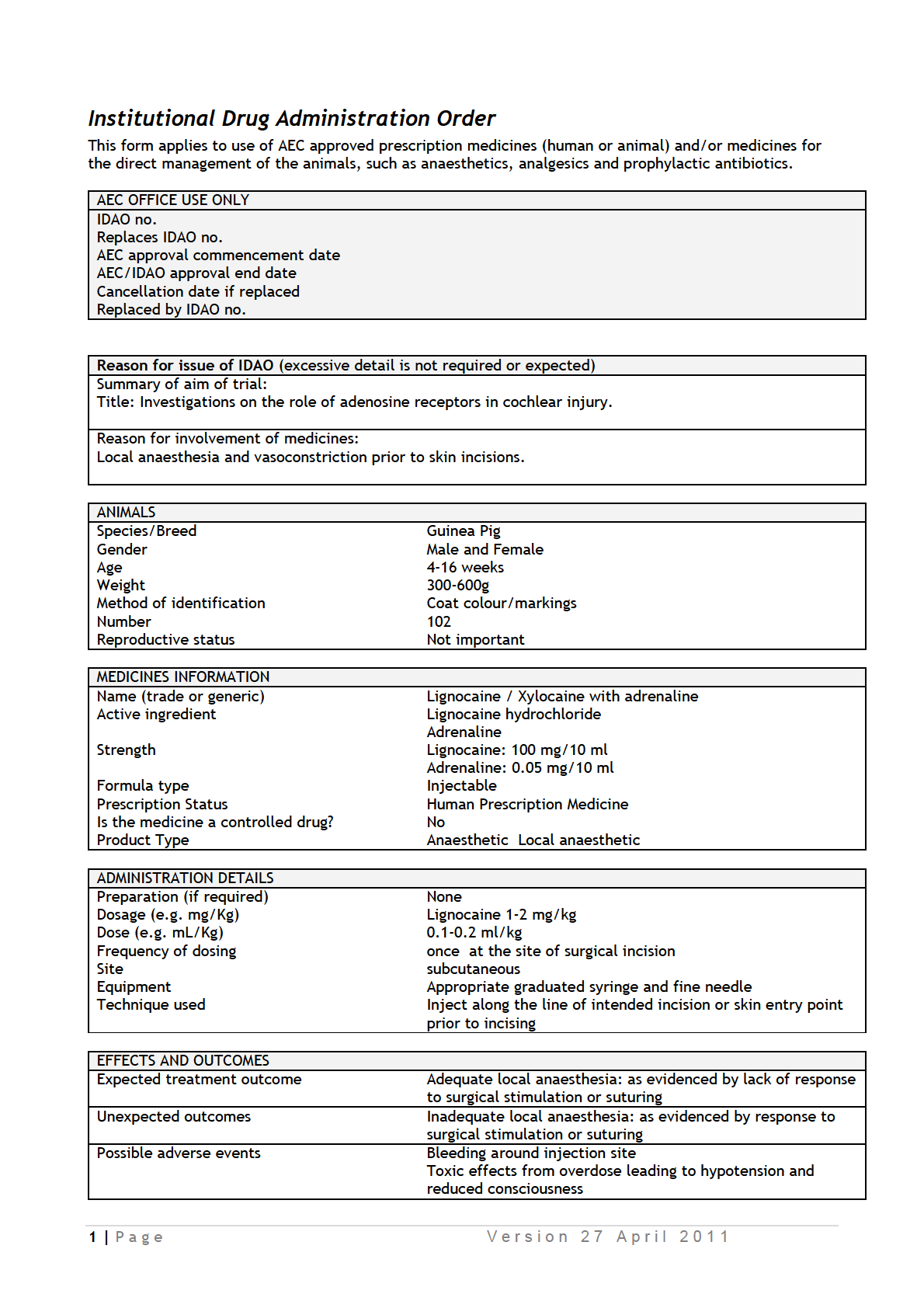
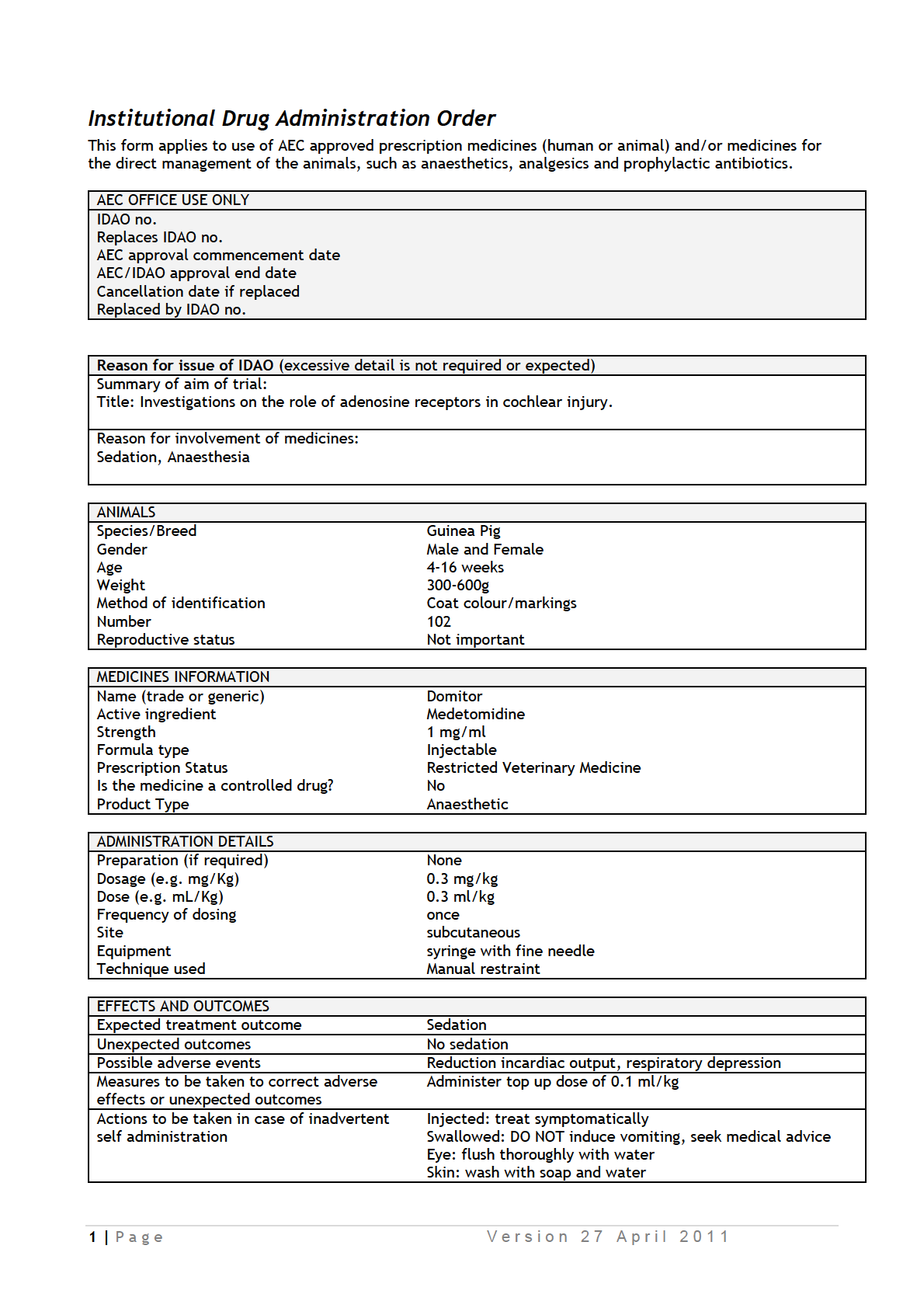
Study 1: Determine the effect of adenosine receptor agonists and antagonists on
cochlear survival after ototoxic injury
In this study, we will determine the effect of adenosine receptor agonists (
) and antagonists
) on hair cell survival in tissue cultures treated with
ototoxic aminoglycoside antibiotic neomycin which selectively depletes outer sensory hair
cells. Exposure to neomycin is used as a model of metabolic stress leading to hair cell death
in this study. Mouse pups (P3-P6) will be killed by decapitation, and cochlear tissues
collected for tissue culture studies. Cochlear explants will be pre-incubated for 19 hours with
and then exposed to neomycin (1 mM) for 3 h at 37°C. Cochlear explants
will be incubated for further 19 hours in culture medium and then fixed with 4% PFA.
Quantitative histology will determine the survival rate of hair cells in whole mounts of the
organ of Corti.
Number of mice required = 25 (Grade A, tissue collection). The number
of animals required for this study is determined by the number of compounds (4) + vehicle
control x 10 cochleae per group = 50 cochleae (25 mice).
Study 2: Determine the effects of adenosine receptor agonists (
) and
antagonists (
) on the development of excitotoxic injury in cochlear
explants
In all studies we will use organotypic tissue cultures of the Wistar rat cochlea at postnatal
day 3-6 (P3-P6) as described previously. Briefly, rat pups will be decapitated and auditory
bullae removed. The cochleae will be decapsulated in ice-cold dissection solution and the
membraneous labyrinth will be separated from the modiolus, and the organ of Corti and
spiral ganglion neurons (SGN) will be kept intact. Cochlear explants will be cultured in
Dulbecco's Modified Eagle Medium and Earle’s Balanced Salt Solution (Thermo Fisher) with
addition of fetal bovine serum and penicillin G. Cochlear explants will be transferred to a cell
culture incubator and maintained at 37°C with 5% CO2 for 24 hours (pre-incubation). To
cause excitotoxic injury, the explants will be exposed to glutamate receptor agonist (NMDA,
0.5 mM) and kainic acid (0.5 mM) for 2 hours (“NK” treatment). This treatment results in loss
of IHC afferent synapses and degeneration of SGN peripheral axons, mimicking excitotoxic
damage caused by noise. The explants will be maintained for further 48 hours in control
culture medium or in culture medium containing
,
or i
. The
explants of the organ of Corti will be then fixed in 4% PFA and prepared for
immunofluorescence and histology. The number of animals required for this study is
80 rats
(Grade A, tissue collection), based on four experimental groups (3 drug-treated and a
vehicle-treated) x 20 animals/group. Additional 5 rat pups are required to establish the NK
model of excitotoxicity in our lab. The number of animals (n = 20/group) is required to obtain
40 cochleae for quantitative analysis of SGN, hair cells and afferent synapses, semi-
quantitative analysis of synaptic swelling and the loss of peripheral axons, and
characterisation of A1 and A2A receptor distribution at the afferent synapses and SGN. This
number has been determined from our previous experience in organotypic culture studies in
the cochlea.
Study 3: Stemming inflammation and fibrosis in the cochlea after cochlear
implantation
Aim 1: Protection of hearing
1.1.
Protecting residual hearing: Guinea pigs will be unilaterally implanted with a
dummy implant along with supraparticles containing different concentrations of
2 weeks after deafening with noise exposure (16kHz, 2hr, 120dBSPL, closed
field). Function will be measured before and weekly up to 8 weeks later and histology will
determine survival of hair cells and neurons at 8 weeks. One ear will be implanted and the
opposite will serve as a non implanted control. Another group of animals will be implanted
[sections 9(2)(b)(ii), 9(2)(i)]
along with empty nanoparticles as a drug delivery control.
Numbers 6 in each group x 4
groups (3 concentrations and 1 control): 24 animals 1.2.
Combination therapy: The A2A agonist (at concentration with maximum effect from
Aim 1.1) will be used alone or in combination with a neurotrophin (BDNF) or another
compound (
) which enhances endogenous adenosine signalling through A1
receptors. After noise exposure deafening procedure, animals will be bilaterally implanted
with supraparticles and one ear will also receive a dummy implant. Function will be
measured each week for 8 weeks, and histology will determine survival of hair cells and
neurons at 8 weeks.
Numbers 6 in each group x 4 groups (3 combinations and 1
control): 24 animals
2.
Aim 2
Effect on Fibrosis: Guinea pigs will be unilaterally implanted with a dummy implant along
with supraparticles containing
(3 different concentrations as above) 2 weeks
after deafening with noise exposure (16kHz, 2hr, 120dBSPL). Two separate measures will
be undertaken to determine the anti-inflammatory action of the
on the
cochlea:
The cochlear vascular permeability will be assessed using Dynamic Contrast MRI (DCE-
MRI). This technique measures the uptake of Gadolinium-based contrast agent (GBCA) into
the cochlea from the circulation, using MRI. The rate of uptake can be used to determine
the vascular permeability in the cochlea, which is a surrogate measure of the inflammatory
response in this tissue. Measurements can be made on the same animal using MRI at 3
different intervals (intervals limited by the need to inject GCBA into the femoral or jugular
vein each time). The fibrosis formed in the cochlea will be quantified and characterised
using µCT and histology. Animals used for MRI (2.1) will be euthanised at 14 or 28 days
after the introduction of the cochlear implant. Tissue will be taken for histology (n=6 at each
interval) or µCT (n=6 at each interval). Histology, using immunohistochemistry for
inflammatory cytokines will indicate the influence of the drugs on inflammatory signalling and
µCT will be used for 3D reconstruction of the electrode and cochlear tissues to assess the
amount of fibrotic tissue around the electrode using our developed techniques for soft-tissue
rendering with µCT. Six additional animals are requested in case of death or surgical
mishaps during the implantation procedure.
Explanation of animal numbers for Aim 2:
As we can only do three MRI timepoints on each animal and one has to be a baseline for
comparison and the last one is terminal because we cannot keep repeating vascular
injections on the same vein we will have 12 experiments running for 14 days and 12
experiments running for 28 days for histology and the same for µCT.(6 animals each time
with
and 6 without). 6 additional animals for contingencies.
Total 54 animals.
3 days and 14 day timepoints have been selected for MRI as these are the times of
maximum inflammation (Bas et al., 2014) and 28 days is selected as the final timepoint to
determine the effect on the fibrotic changes.
Procedure
Baseline MRI
Day 3 MRI
Day 14
Day 28
uCT
Reg
X
X
6
Reg
X
6
Control
X
X
6
Control
X
6
Histology
Reg
X
X
6
Reg
X
6
Control
X
X
6
Control
X
6
Appendix 2
EForm Name: AE and Bio-Safety Form v4
Page:
Section G: Attachments
Section:
Please list all attachments appended in support of this application:
Question:
File Name:
1986_personnel.pdf
Appendix 3
EForm Name: AE and Bio-Safety Form v4
Page:
Section G: Attachments
Section:
Please list all attachments appended in support of this application:
Question:
File Name:
Monitoring sheet.xlsx
Animal W
Species
Guinea Pig
Experiment:
Animal #
Surgeon:
Experiment day
Day 1
Day 2
Day 3
Date
Day
Time
ON OBSERVATION
Activity (normal/active/alert/inactive/lethargic)
Posture (normal/hunched)
Coat (normal/rough)
Breathing (normal/regular/rapid/laboured/shallow)
Skin turgor
ON HANDLING
Inquisition
Diarrhoea
Vocalisation
Signs of dehydration
CNS signs (seisures/convulsions)
BODY WEIGHT
Measured weight
Weight loss (gms)
Weight loss (%)
FOOD AND WATER
Food intake ok
Weight of full bottle
Weight of bottle today
Fluid intake
WOUND SITE
Okay/clean
Bleeding
Other discahrges/infection
Suture/clips Ok
POST-OP SUPPORT
Name of analgesic
Dose
Other
Signature
Comments
Perform euthanasia if weight loss is 15% of start
Humane endpoints
Welfare Record Sheet
Cochlear implant
Date of expt:
AEC #
Day 4
Day 5
Day 6
Day 7
Day 8
Day 9
t body weight or greater.
Day 10
Animal Welfare Monitoring Record (no abnormalities found)
Species:
Date of treatment/surgery:
AEC #:
Number of animals:
Animal identifications:
Researcher name:
A/h contact:
RI name:
A/h contact:
Instructions for animal technicians:
Date
Time
No abnormalities
Initials
Date
Time
No abnormalities
Initials
√
√
Appendix 4
EForm Name: AE and Bio-Safety Form v4
Page:
Section G: Attachments
Section:
Please list all attachments appended in support of this application:
Question:
File Name:





Appendix 7
EForm Name: AE and Bio-Safety Form v4
Page:
Section G: Attachments
Section:
Please list all attachments appended in support of this application:
Question:
File Name:
001986_Animal Experimental Timeline.docx
Animal Experimental Timeline (AEC# 001986)
The following is the timeline for the experiments described
Study 3 – Aim 1a – Protection of hearing – determining optimal drug dose
Animal numbers: 24 [4 groups (3 drug concentrations and 1 control). 6 animal per group.]
Timeline: (for a representative animal in one group)
Day 1 -
Baseline Auditory Brainstem Response (ABR) and noise exposure to both ears
Day 14 -
Anaesthetise animals and perform ABR to confirm permanent hearing loss followed by
cochlear implant surgery on one ear along with supraparticles with or without drugs.
The other ear will not be operated and serve as noise control.
Day 28 -
ABRs on both ears (2 weeks post implantation)
Day 42 -
ABRs on both ears (4 weeks post implantation)
Day 56 -
ABRs on both ears (6 weeks post implantation)
Day 70 -
ABRs on both ears (8 weeks post implantation) and euthanise the animal, collect tissues
for histology.
Study 3 – Aim 1b – Protection of hearing – drug combination
Animal numbers: 24 [4 groups (3 drug combinations and 1 control). 6 animal per group.]
Timeline: (for a representative animal in one group)
Day 1 -
Baseline ABR on and noise exposure to both ears
Day 14 -
ABR to confirm permanent hearing loss followed by cochlear implant surgery along with
supraparticles with or without drugs.
.
Day 28 -
ABRs on both ears (2 weeks post implantation)
Day 42 -
ABRs on both ears (4 weeks post implantation)
Day 56 -
ABRs on both ears (6 weeks post implantation)
Day 70 -
ABRs on both ears (8 weeks post implantation) and euthanise the animal, collect tissues
for histology.
Study 3 – Aim 2 – Effect of fibrosis
Animal numbers: 54 [8 groups (2 timelines of 3 drug concentration and 1 control). 6 animal per
group, plus 6 additional animal for contingency.]
Timeline: (for a representative animal in one group)
Day 1 -
Baseline MRI on guinea pig
Day 3 -
Baseline ABR and noise exposure to one ear
Day 17 -
ABR to confirm permanent hearing loss followed by cochlear implant surgery along with
supraparticles with or without drugs. The other ear will not be operated and serve as
control.
Day 31 -
MRI on guinea pig (2 weeks post
implantation). The animal will be euthanised
and tissue collected for µCT and histology.
This will end the expts on one group with 14
day timeline.
Day 45 -
MRI on G.pig (4 weeks post implantation). The animal
will be euthanised and tissue collected for µCT and
histology. This will end the expts on one group with 28
day timeline.
Appendix 8
EForm Name: AE and Bio-Safety Form v4
Page:
Section G: Attachments
Section:
Please list all attachments appended in support of this application:
Question:
File Name:
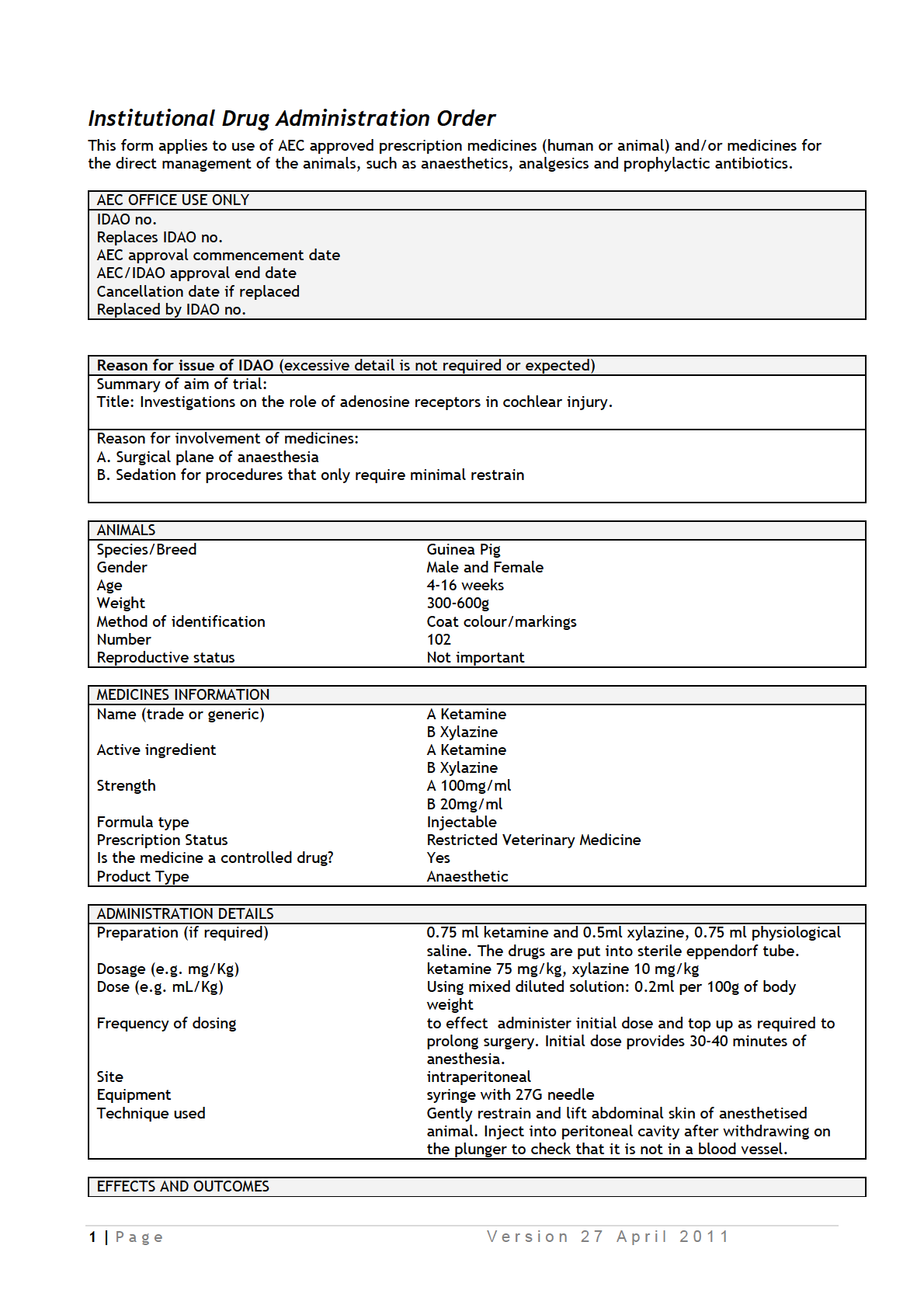
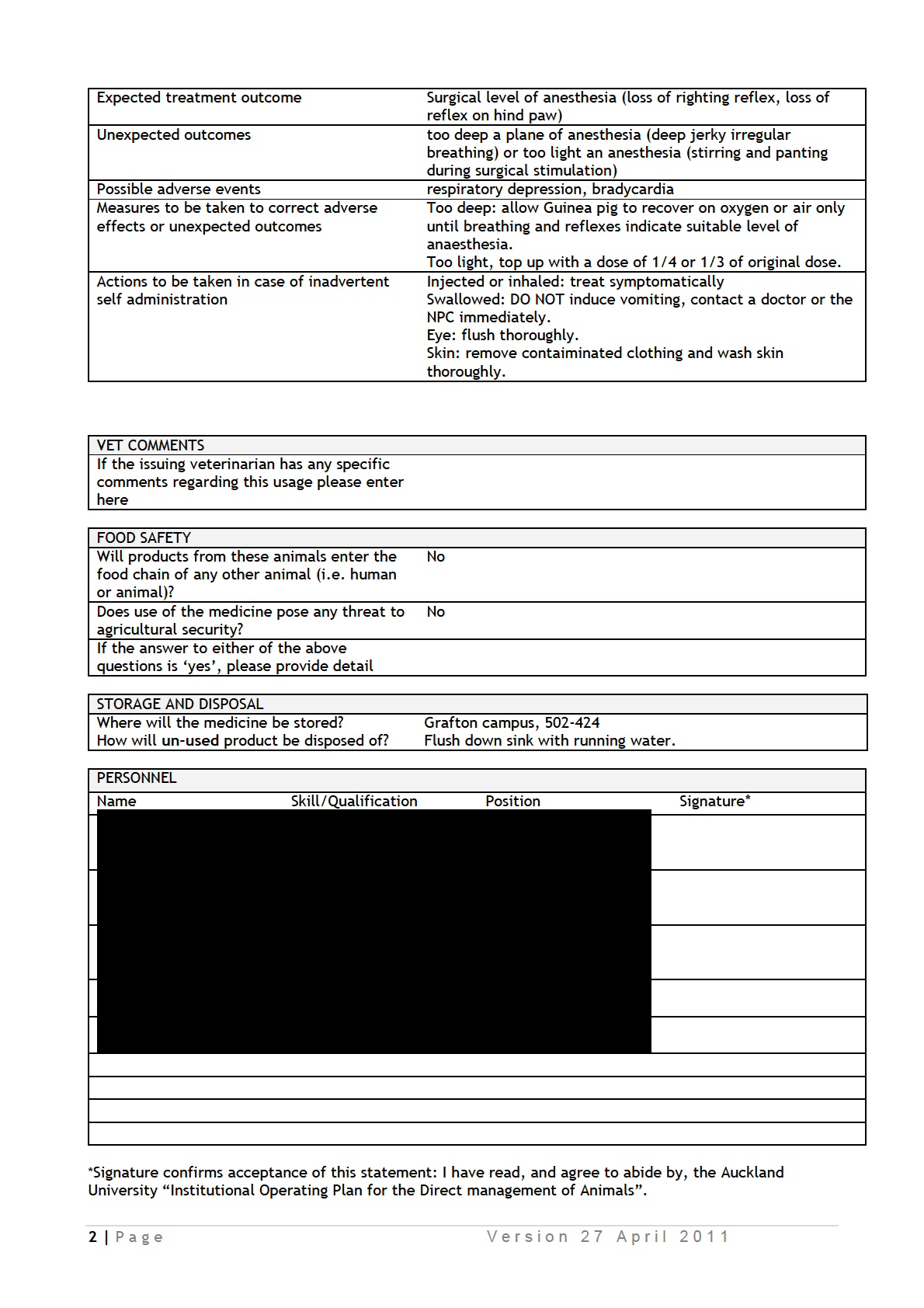
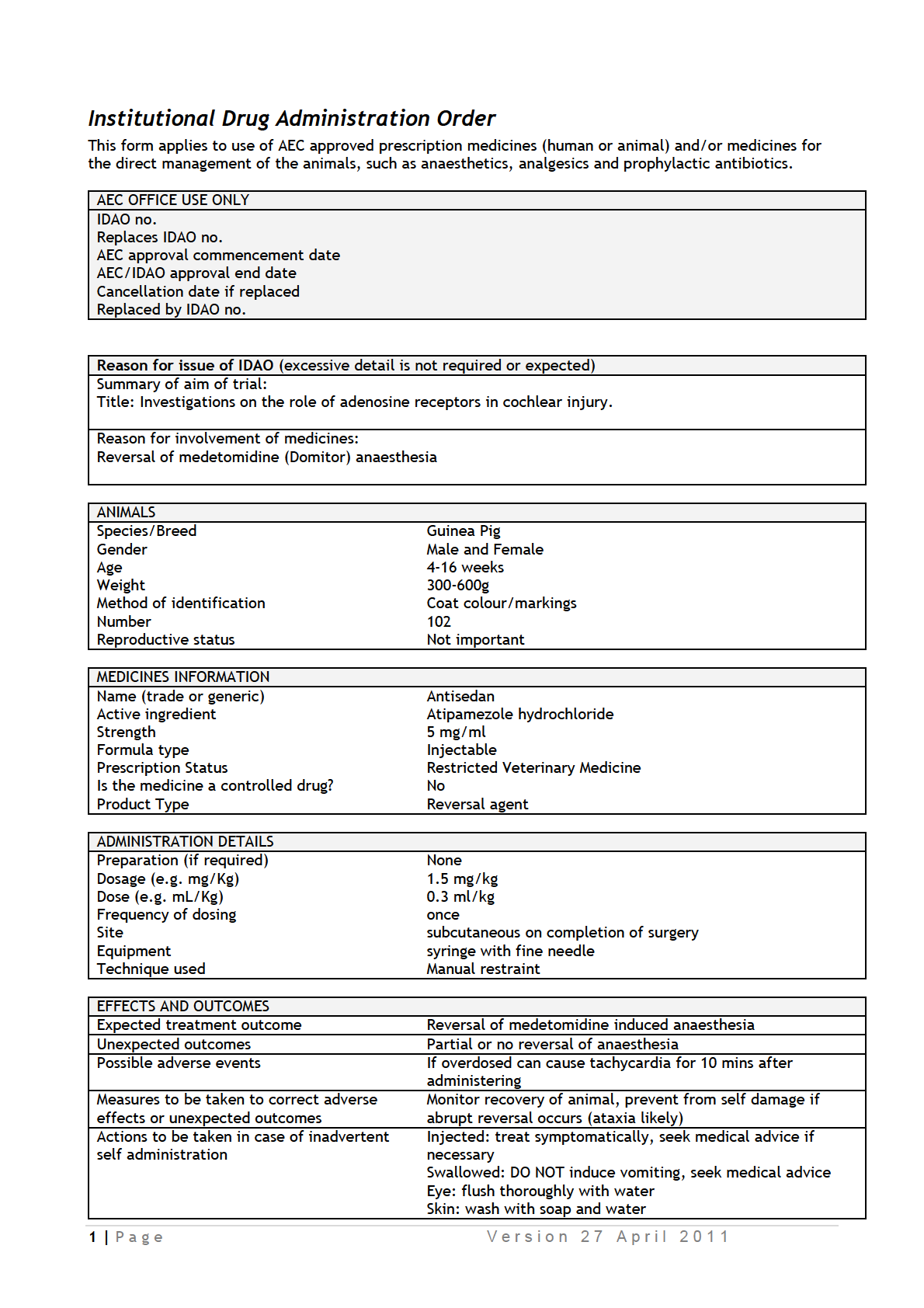
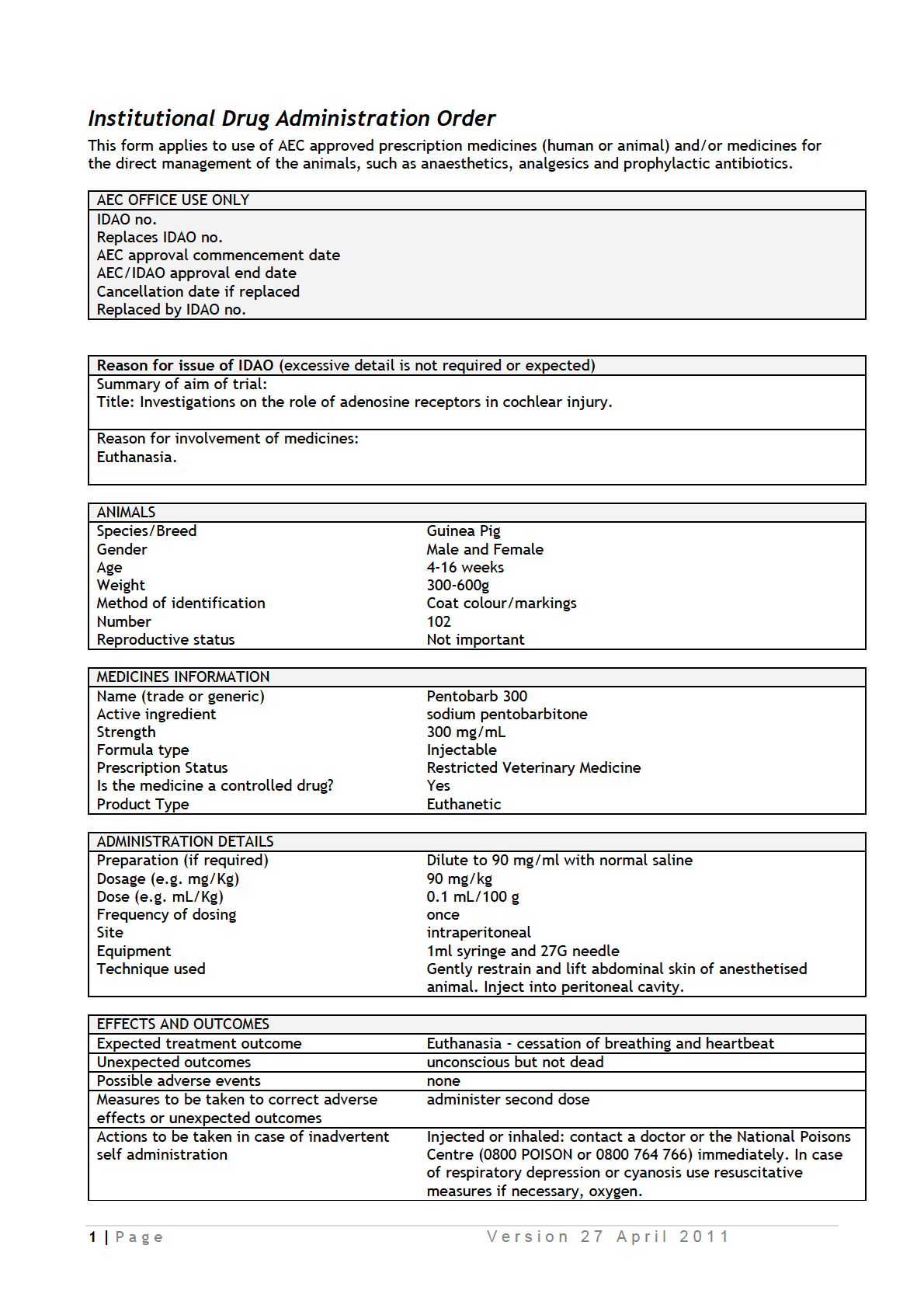
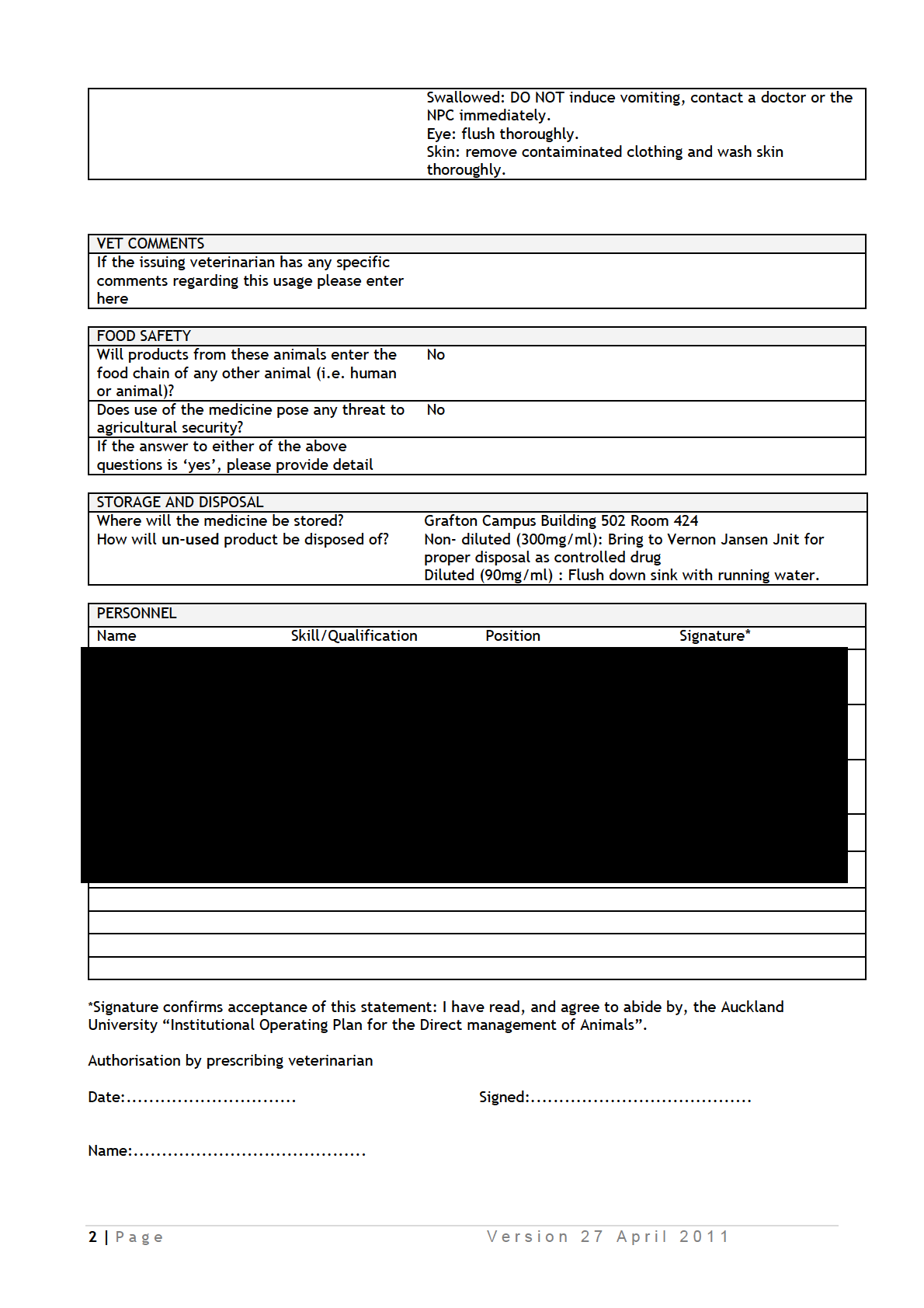
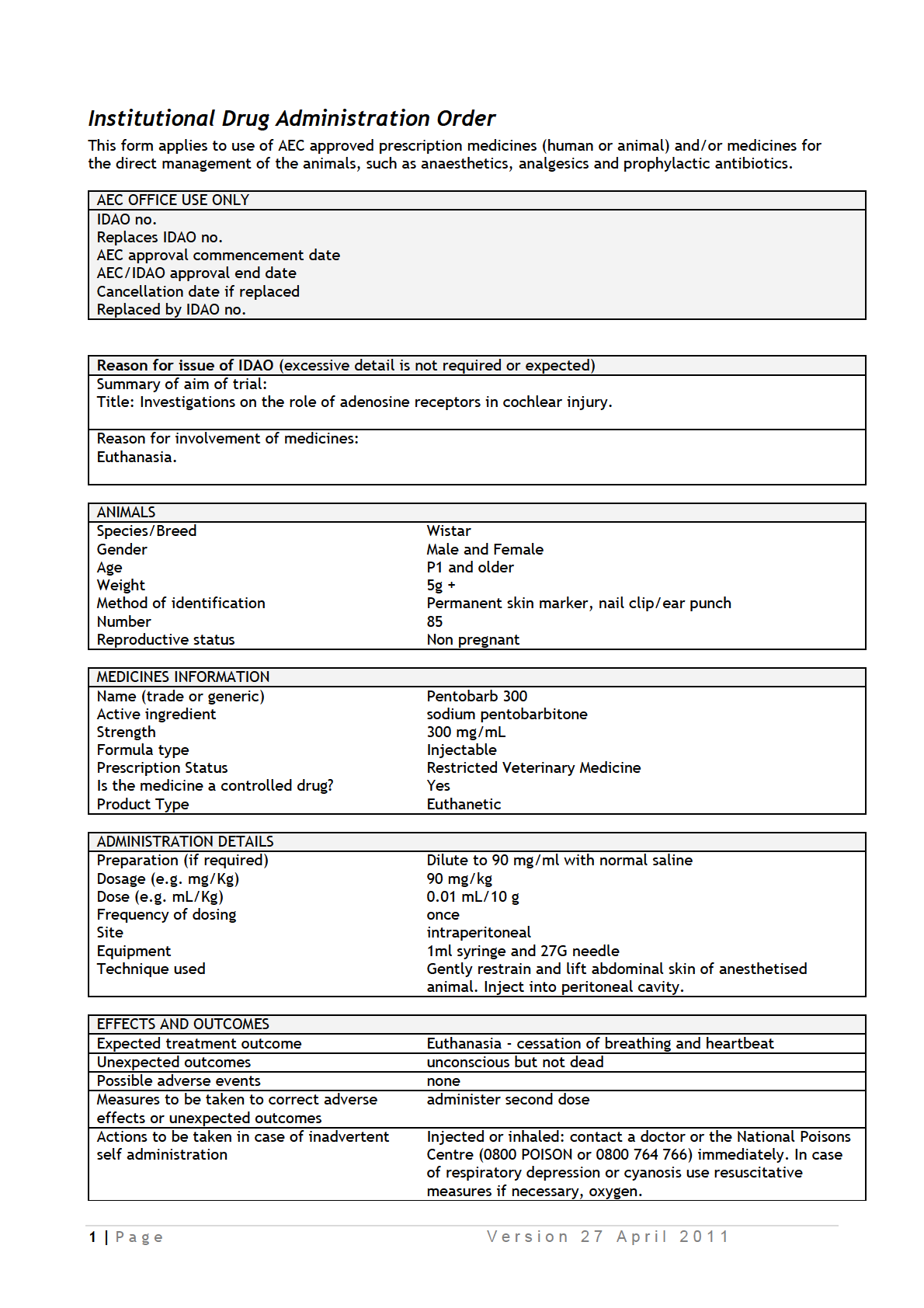
IDAOs for AEC 001986.pdf
Authorisation by prescribing veterinarian
Date:..............................
Signed:.......................................
Name:.........................................
3 | P a g e
V e r s i o n 2 7 A p r i l 2 0 1 1
*Signature confirms acceptance of this statement: I have read, and agree to abide by, the Auckland
University “Institutional Operating Plan for the Direct management of Animals”.
Authorisation by prescribing veterinarian
Date:..............................
Signed:.......................................
Name:.........................................
3 | P a g e
V e r s i o n 2 7 A p r i l 2 0 1 1

*Signature confirms acceptance of this statement: I have read, and agree to abide by, the Auckland
University “Institutional Operating Plan for the Direct management of Animals”.
Authorisation by prescribing veterinarian
Date:..............................
Signed:.......................................
Name:.........................................
3 | P a g e
V e r s i o n 2 7 A p r i l 2 0 1 1

*Signature confirms acceptance of this statement: I have read, and agree to abide by, the Auckland
University “Institutional Operating Plan for the Direct management of Animals”.
Authorisation by prescribing veterinarian
Date:..............................
Signed:.......................................
Name:.........................................
3 | P a g e
V e r s i o n 2 7 A p r i l 2 0 1 1

Appendix 9
EForm Name: AE and Bio-Safety Form v4
Page:
Section G: Attachments
Section:
Please list all attachments appended in support of this application:
Question:
File Name:
001986 MEMO_2.docx
List of additional corrections and responses to the AEC reviewers’ comments for
AEC 001986
1. Please ensure
and
contact
to
complete module 2a and 2b or be signed off as competent before they
handle any animals.
We have been unable to contact
as he is on leave and will come
back next month. We have been in touch with
and have emailed
him with a request to check records whether we have to undergo this
training. If required he will enrol us both on the training in next slot but
these are not held very frequently (and are often oversubscribed). We
are very concerned that the project (and student progress) will be held up
until the training is complete.
is not involved in working with
animals and is happy to agree not to be involved with any animal work, or
to remove himself from the ethics approval completely until the training is
complete, so the work can proceed.
has been involved in the
same experimental animal research for many years under previous ethics
approvals and we ask that he be allowed to continue to do this work while
waiting for his records to be assessed and for him to be signed off by
as competent or to complete the modules.
2. You state you will give 15 ml/kg during anaesthetic after surgery, but
the SOP attachment says "Before the scanning procedure, saline solution
will be injected subcutaneously (20 ml/kg) to avoid dehydration during
the scanning procedure". Please amend this or clarify why you have
different dose rates.
This saline dose has been amended in the SOP (now referred to as
“additional information”, see next question).
3. You cannot have a document that is called an SOP unless it has been
approved by the AEC. Please either completely remove attachment 7
(AEC SOP) and ensure this information is included within the text of the
application, or rename this attachment e.g. ‘Additional Information’.
Please keep in mind that attachments should add value or additional
information to what is already in the application.
This SOP file is now referred to as “additional information”. The reference
to this file in D.4a first line is amended to “See appendix (additional
information) for details…”. We have included this to provide details on
procedures which we cannot provide in the space provided for the text
(and were asked for this after the application screening)
4. Please do not mention the name of drugs in your application or within
attachments (particularly attachment 7). You should refer to IDAO’s for
the use of drugs (For example, “anaesthesia as per IDAO”).
The attachment 7 now referred as “Additional information” has been
amended so does not mention any drug name, except for Gadolinium-
DPTA which is a standard MRI contrast agent.
5. Section A: As per provision 20 of the previous outcome letter, please
remove the sentence “one of our investigators is a veterinary surgeon”.
All people using the title veterinarian in New Zealand must be registered
with the Veterinary Council of NZ and hold a current practicing certificate.
We cannot find reference to this anywhere in the documents.
6. D13. Replacement: Please add that you assessed the possibility of
using non-sentient or non-living alternatives using your sources (insert
your sources) and there were no alternatives.
We have provided additional information in D13c as below:
Replacement: We are very interested in replacement approaches
(especially for Study 1 and 2) and have assessed, through a search of the
literature (PubMed and Medline), the possibility of using non-sentient or
less sentient (zebrafish) animals and
in silico or immortal cell line
approaches for this work. Zebrafish are used substantially to screen for
ototoxic drugs (eg Ou et al., Drug Discov Today, 15(7-8): 265–271.
doi:10.1016/j.drudis.2010.01.001) but we have not yet assessed whether
these would be suitable as alternatives to study otoprotective
mechanisms and it is often required to repeat these studies in mammalian
species as a proof-of-principle. There are several immortal cochlear
sensory hair cell lines (Rivolta and Holley, 2002 J Neurobiol. 53:306-18),
which have been used to look at ototoxicity metabolic mechanisms. But
these lack the integrity of the sensory epithelium necessary to look at the
interaction of supporting and sensory tissues, and needed for this study
(the supporting cells are considered to be involved in organising sensory
cell death). Furthermore, some of the aminoglycoside ototoxic pathways
in immortal cochlear sensory hair cell lines are different to those seen
in
vivo (Chen et al., 2012, Hearing Research 284:33-41). However, we
have developed systems for partial replacement and are using
organotypic cultures of the inner ear where possible, such as in the first
two studies described in this proposal. These are taken from neonatal
(P3-P6 mice) and do not involve any experimental manipulation of the
animal. The studies of cochlear implantation (Study 3) require
in vivo
experiments in order to assess the natural immune response and
formation of the fibrosis as it occurs in human surgical implantation. It is
not possible to use cell lines for these particular research questions as
these do not mimic the complex relationship between the different
sensory, neural and secretory tissues involved in the cochlear response to
injury. However, we are investigating ways to model the local changes in
the inner ear following surgery (ie those not involving a systemic
response or are confined to signal transduction pathways expressed in cell
lines) to evaluate the impact of treatments using cell culture or in vitro
systems in a similar way to previous studies (eg Bas et al., 2015,
Frontiers of Cellular Neuroscience doi: 10.3389/fncel.2015.00303, and
our previous studies Vlajkovic et al. (1998) Hear Res.117:71-80). The
technology for developing inner ear organoids (Koehler et al., Nature
Biotechnology 35, 518–520 (2017) doi:10.1038/nbt.3899), may
eventually allow study of localised tissue responses and mechanisms of
injury.
In the attachments – the attachment no 1 (AEC 1986 supplementary
details on design and methods) seems to be wrong. It contains the
information on personnel instead.
The wrong file was uploaded by mistake. Thank you. This has been
changed. The file includes details on the procedures for the experiments
that could not be included in the text space and give more detailed
information to the committee..
Appendix 10
EForm Name: AE and Bio-Safety Form v4
Page:
Section G: Attachments
Section:
Please list all attachments appended in support of this application:
Question:
File Name:
AEC 1986 Additional information.docx
Additional information for operating procedures
Preparation of tissues for organotypic cultures
Mouse and rat pups (P3-P6) will be euthanised by decapitation, and cochlear tissues will be
collected for tissue culture studies.
Procedures for in vivo experiments on guinea pigs, including measurements of auditory brain
response (ABR), surgery of cochlear implantation (CI) and post-operation monitoring, and
MRI scanning.
1. Procedures of ABR measurements
Animals will be anaesthetised as per IDAO, and then placed onto a heating pad to maintain
body temperature at 37C. ABRs were obtained by placing fine platinum electrodes
subdermally at the mastoid region of the ear of interest (active electrode), scalp vertex
(reference) and mastoid region of the opposite ear (ground electrode). The acoustic stimuli
for ABR were produced and the responses recorded using a Tucker Davis Technologies
auditory physiology System 3 workstation (Alachua, FL, USA). A series of pure tone pips (5
ms duration, 1.5 ms rise and fall times, 1-32 kHz) are presented at varying intensity (10-90
dB SPL).
Animals will be allowed to recover in a dimmed area with warming blanket prior to returning
to VJU housing. The duration of each ABR measurement is around 40 minutes. The same
animal will have a maximum of three ABR measurements in total with intervals of 2-4 weeks
according to allocated study groups.
2. Procedures of CI surgery and post-operation monitoring
The Guinea pigs will be anesthetized as per IDAO. The animal will also receive analgesic as
per IDAO. The surgical area is shaved and aseptically prepared with 70% ethanol and
povidone-iodine. Local anesthetic will be injected along the intended incisions, as per IDAO.
The subjects will be placed on a heating pad and body temperature maintained around
37°C. The eyes will be instilled with lubricating gel/liquid.
Surgery will be performed as follows under a surgical stereo microscope. Through a left
post-auricular incision, the left bulla will be exposed and opened using a 2 mm steel burr and
bone forceps to gain access to the round window niche. The basal turn is cleared off any
connective tissue and underneath bone exposed. A cochleostomy will be made just inferior
and anterior to the round window using a 0.5 mm steel burr. Through the cochleostomy, the
CI electrode array is inserted into the scala tympani until slight resistance was felt. The
cochleostomy will be sealed with muscle plugs. The bulla opening will then be sealed with
bone pieces and dental cement. The skin will be closed with surgical sutures and skin
adhesive.
Animals will be monitored daily post-operation until Day 10 using the customised ‘monitoring
sheet’ and then will be monitored weekly until the end of each experiment (up to 8 weeks
according to the study plan).
3. Procedures of animal preparation for MRI scanning
The Guinea pigs will be sedated as per IDAO and transferred to inhalant anesthetic
chamber. The anesthesia will be induced as per IDAO. The animal will then be transferred to
HFMRI (Varian 4.7T) coil chamber and anesthesia maintained using an appropriate face
mask throughout the MRI procedure for a maximum of 2 hrs period.
If the animal shows sign of irritation or distress, it will be alternatively anesthetized with
injectable anaesthetics as per IDAO. Supplemental doses of anaesthetics may be given
during the procedure as necessary.
Before the scanning procedure, saline solution will be injected subcutaneously as per
protocol to avoid dehydration during the scanning procedure. Throughout the MRI scanning
procedure core body temperature was monitored with a rectal probe and maintained with
warm air and heart rate/respiration was monitored continuously. The eyes will be instilled
with lubricating gel/liquid.
For injecting the contrast agent the protocol will be as follows:
The animal will receive analgesic as per IDAO. The surgical area, inner thigh region of hind
legs will be shaved and aseptically prepared. The subjects will be placed on a heating pad
and body temperature maintained around 37°C.
An incision, about 1cm long, will be made just over the femoral region and vein exposed.
Animal will then be slowly injected with warm contrast agent Gadolinium DTPA (1.5
mmol/kg, @ 1ml/min) using a fine 30G needle. The wound will be sutured and sealed with
skin adhesive.
The animal will then be placed back into the HFMRI chamber and recordings done.
Some animals will recover at the end of the scans for re-imaging one or more weeks later
(as per experimental methods) following the same method to investigate the chronic
changes in cochlea permeability in same animal. A maximum of three scanning procedures
will be performed for each animal. After completion of the final MRI scan, the animals will be
euthanized and tissues harvested for histochemistry procedures.














































