
133 Molesworth Street
PO Box 5013
Wellington 6140
New Zealand
T +64 4 496 2000
W www.medsafe.govt.nz
6 August 2021
Tracy Livingston
By email: [FYI request #14927 email]
Ref:
H202106580
Dear Tracy
Response to your request for official information
Thank you for your request under the Official Information Act 1982 (the Act) to the Ministry of
Health (the Ministry) on 27 May 2021. In response to your earlier request (H202102975 refers),
you asked for:
“I requested the Iatrogenic reports from 2019 and 2020, (#14927) which were denied
because I asked for the 'unscrubbed' data. I am sorry that I may have requested wrongly.
What I mean was, the data without personal information but that included all the iatrogenic
deaths reported (and obviously the medicines involved) and none missed out or deleted.”
On 18 June 2021, the Ministry extended the timeline to respond to your request under section
15A of the Act until 20 August 2021 due to the need for further collation, research and
consultation.
Please find attached the information requested. The data for deaths in 2019 is an excerpt from a
report to the Medicines Adverse Reactions Committee and some information has been withheld
under section 9(2)(a) of the Act to protect the privacy of natural persons. The data for deaths in
2020 has specifically prepared by the New Zealand Centre for Adverse Reactions Monitoring
(CARM) at the University of Otago to respond to your request. CARM undertakes
pharmacovigilance in New Zealand under contract to the Ministry. The report is released to you in
full.
Accompanying the information for 2020 is a statement from the Director of the New Zealand
Pharmacovigilance Centre at CARM that outlines important caveats that must be considered
when interpreting the data. Similar caveats should be applied to the information provided for
2019. It is also important to note that while anyone – doctors, nurses, pharmacists,
pharmaceutical companies, government agencies or a member of the public – can report an
adverse reaction to CARM, reporting is voluntary. There is more information about adverse
reaction reporting at:
https://nzphvc.otago.ac.nz/reporting/
I also noted in your original request an interest in CARM reports related to Sudden Infant Death
Syndrome (SUDI). SUDI cases are often investigated by coroners as sudden or unexplained
deaths and many coronial findings are published on the New Zealand Legal Information Institute
website at:
www.nzlii.org/nz/cases/NZCorC/ (search on SUDI or “cot death”). The Chief Coroner
has also issued a
Recommendations Recap that specifically examines SUDI deaths at:
www.coronialservices.justice.govt.nz/assets/Documents/Publications/issue-14-recommendations-
recap2.pdf

I trust this information fulfils your request. Under section 28(3) of the Act you have the right to ask
the Ombudsman to review any decisions made under this request. The Ombudsman may be
contacted by email at:
[email address] or by calling 0800 802 602.
Please note that this response, with your personal details removed, may be published on the
Ministry website at:
www.health.govt.nz/about-ministry/information-releases/responses-official-
information-act-requests.
Yours sincerely
Chris James
Group Manager
Medsafe
Page 2 of 2
 New Zealand Pharmacovigilance Centre
University of Otago
New Zealand Pharmacovigilance Centre
University of Otago
Email: [email address]
Website: https://nzphvc.otago.ac.nz
CAVEAT DOCUMENT
Accompanying statement to data released from the
NEW ZEALAND CENTRE FOR ADVERSE REACTIONS MONITORING
1982
The Centre for Adverse Reactions Monitoring (CARM) has only limited details about each
suspected adverse reaction contained in its Database. It is important that the limitations and
ACT
qualifications which apply to the information and its use are understood.
The data made available represent the collection of spontaneous reports in the CARM database
associated with therapeutic products/vaccines granted regulatory approval for use in New Zealand.
Reports have been submitted to the Centre since April 1965 and in many instances describe no
more than suspicions which have arisen from observation of an unexpected or unwanted event.
This level of reporting is due to CARM encouraging reporters to report events they suspect may be
associated with a pharmaceutical product/vaccine irrespective of whether or not they believe it was
the cause. CARM accepts all reports and proof of causality is not required when submitting a
report to CARM. Coincidental events that may be unrelated to pharmaceutical product/vaccine
exposure may be reported. This is particularly possible when the product has widespread use, or
is used in targeted strategies such as vaccination campaigns.
INFORMATION
In most instances it cannot be proven that a pharmaceutical product or ingredient is the cause of
an event in the Database. Reports vary in quality, completeness and detail and may include detail
that is incorrect. Consequently, a report in the CARM database of an event does not confirm that
the pharmaceutical product/vaccine caused the event.
The volume of reports for a particular product may be influenced by the extent of use of the
product, publicity, nature of reactions and other factors which vary over time and from product to
OFFICIAL
product. It is generally accepted internationally that systems such as CARM are subject to under-
reporting which may result in scant reports for events perceived by the reporter to be minor or well
recognised, whilst more serious or unexpected events are possibly more likely to be reported, even
if they are coincidental. Moreover, no information is provided on the number of patients exposed to
THE
the product.
The data contained in these tables are further subject to ongoing internal quality controls, review
and updating and therefore may be subject to change, particularly if follow-up information is
received.
For the above reasons interpretations of adverse reaction data, and particularly those based on
UNDER
comparisons between pharmaceutical products, may be misleading. Any use of this information
must take into account at least the above. Although this information is now released, it is strongly
recommended that prior to any use of such information, CARM is contacted for interpretation.
Any publication, in whole or in part, of the obtained information must have published with it a
statement:
(i)
of the source of the information
(ii)
that the information is not homogenous at least with respect to origin or likelihood
RELEASED that the pharmaceutical product/vaccine caused the adverse reaction,
(iii)
that the information does not represent the opinion of the NZPhvC or CARM.
Director
New Zealand Pharmacovigilance
 New Zealand Pharmacovigilance Centre
University of Otago
New Zealand Pharmacovigilance Centre
University of Otago
Email: [email address]
Website: https://nzphvc.otago.ac.nz
Fatal Cases reported to CARM from 01January to 31 December 2020
This listing covers the cases reported in 2020 which resulted in a fatal outcome regardless of causality. There were 107 reports, identifying 129 suspect medicines.
An * (asterisk) beside the medicine name indicates a suspect medicine. Some cases involved more than one suspect medicine.
Report No
Date
Gender
Age
Medicines/Vaccine
Reactions
135633 JAN2020
Female
42 *
AMPHOTERICIN,
LIPOSOMAL
HYPERKALAEMIA
1982
*
GABAPENTIN
CARDIAC
ARREST
* METHADONE
PIPERACILLIN/TAZOBACTAM
MULTIVITAMINS
ACT
135703
JAN2020
Female
53
* INFLIXIMAB
RENAL FAILURE ACUTE
135764 JAN2020
Male
82 *
ELTROMBOPAG
MYOCARDIAL
INFARCTION
FELODIPINE
THROMBOCYTOSIS
CANDESARTAN
ARM
PAIN
METFORMIN
CHEST
PAIN
CITALOPRAM
135869 JAN2020
Male
55 *
CLOZAPINE
MYOCARDIAL
ISCHAEMIA
135870 JAN2020
Male
29 *
CLOZAPINE
SUICIDE
INFORMATION
DIAZEPAM
OMEPRAZOLE
MYLANTA
135871 JAN2020
Male
50 *
CLOZAPINE
PNEUMONIA
MORPHINE SULPHATE
CHRONIC OBSTRUCT AIRWAYS DISEASE
PREDNISONE
OFFICIAL
135872 JAN2020
Male
84 *
CLOZAPINE
PNEUMONIA
135873 JAN2020
Male
68 *
CLOZAPINE
PARKINSONISM
LEVODOPA/CARBIDOPA
(100/25) ASPIRATION PNEUMONITIS
THE
CODEINE
FLUDROCORTISONE
LORAZEPAM
135874 JAN2020
Female
68 *
CLOZAPINE
ARRHYTHMIA
MIDAZOLAM
PARKINSONISM
ACETYLSALICYLIC
ACID
DEMENTIA
MADOPAR
TRANSIENT ISCHAEMIC ATTACK
UNDER
ROPINIROLE
SYNCOPE
135875 JAN2020
Male
81 *
CLOZAPINE
DEMENTIA
135876 JAN2020
Female
67 *
CLOZAPINE
MYOCARDIAL
ISCHAEMIA
CORONARY ARTERY DISORDER
135877 JAN2020
Male
58 *
CLOZAPINE
PARKINSONISM
MYOCARDIAL
INFARCTION
RELEASED
PNEUMONIA
135878
JAN2020
Female
68
* OCTREOTIDE LAR
COLLAPSE CIRCULATORY
HEART
DISORDER
NZPhvC – Deaths January to December 2020
1
 New Zealand Pharmacovigilance Centre
University of Otago
New Zealand Pharmacovigilance Centre
University of Otago
Email: [email address]
Website: https://nzphvc.otago.ac.nz
Report No
Date
Gender Age
Medicines/Vaccine
Reactions
135879 JAN2020
Male
61 *
VIEKIRA PAK-RBV
CHOLELITHIASIS
SALBUTAMOL
DYSPNOEA
FATIGUE
MYOCARDIAL
ISCHAEMIA
CORONARY ARTERY DISORDER
1982
135906 JAN2020
Male
43 *
CLOZAPINE
CARDIAC
ARREST
135907 JAN2020
Male
77 *
CLOZAPINE
APPENDICITIS
ACT
LORATADINE
ASPIRATION
TERAZOSIN
LAXSOL
135908 JAN2020
Male
54 *
CLOZAPINE
MEGACOLON
ACQUIRED
STROKE
135909 JAN2020
Male
38 *
CLOZAPINE
SUDDEN
DEATH
135910 JAN2020
Male
40 *
CLOZAPINE ASPIRATION
PNEUMONITIS
LEVOMEPROMAZINE BOWEL
MOTILITY
DISORDER
DIAZEPAM
HYOSCINE
TOPICAL
INFORMATION
VALPROATE
SODIUM
135911 JAN2020
Male
66 *
CLOZAPINE
PNEUMONIA
135912 JAN2020
Female
64 *
CLOZAPINE
MELANOMA
MALIGNANT
135913 JAN2020
Male
56 *
CLOZAPINE
CONSTIPATION
OFFICIAL
DIAZEPAM
RECTAL
ULCER
LORAZEPAM
INTESTINAL
PERFORATION
PAROXETINE
PERITONITIS
PROPRANOLOL
THE
135914 JAN2020
Male
48 *
CLOZAPINE
INTENTIONAL
OVERDOSE
135915 JAN2020
Male
67 *
CLOZAPINE
ASPIRATION
INTESTINAL
OBSTRUCTION
RESPIRATORY
ARREST
RENAL FAILURE AGGRAVATED
UNDER
135916 JAN2020
Female
64 *
CLOZAPINE
HYPERTENSION
HEART
DISORDER
LIVER
FATTY
HEPATIC
CIRRHOSIS
135917 JAN2020
Male
58 *
CLOZAPINE
CARDIAC
ARREST
135918 JAN2020
Female
75 *
CLOZAPINE
CARDIAC
FAILURE
RELEASED
DIABETES
MELLITUS
135982 FEB2020
Male
43 *
CLOZAPINE
MEGACOLON
ACQUIRED
SEPSIS
SECONDARY
COLITIS
NZPhvC – Deaths January to December 2020
2
 New Zealand Pharmacovigilance Centre
University of Otago
New Zealand Pharmacovigilance Centre
University of Otago
Email: [email address]
Website: https://nzphvc.otago.ac.nz
Report No
Date
Gender Age
Medicines/Vaccine
Reactions
136017 FEB2020
Female
91 *
RIVAROXABAN
CEREBRAL
HAEMORRHAGE
ACE INHIBITOR (NOS)
THROMBOSIS CEREBRAL
THYROXINE
PARACETAMOL
UNCLASSIFIED
AGENT
1982
136027 FEB2020
Female
64 *
ATEZOLIZUMAB
LYMPHANGITIS
PULMONARY
INFILTRATION
ACT
136028 FEB2020
Male
29 *
ATEZOLIZUMAB
FEVER
*
COBIMETINIB
FATIGUE
PROGRESSION OF DISEASE
136172
FEB2020
Male
50
* PEMBROLIZUMAB
PROGRESSION OF DISEASE
136174 FEB2020
Male
67 *
VALSARTAN/SACUBITRIL SUDDEN
DEATH
136250 MAR2020
Female
83 *
FLUCLOXACILLIN
JAUNDICE
RIVAROXABAN
HEPATITIS
CHOLESTATIC
BETAMETHASONE
PARACETAMOL
METOPROLOL
INFORMATION
136300 MAR2020
Male
63 *
DAUNORUBICIN
CARDIOMYOPATHY
*
ANTHRACYCLINE
NOS
*
DAUNORUBICIN
136332 MAR2020
Female
35 *
LAMOTRIGINE
CONVULSIONS
LEVETIRACETAM
THERAPEUTIC RESPONSE DECREASED
OFFICIAL
136365
MAR2020
Female
69
* POMALIDOMIDE
PROGRESSION OF DISEASE
136416 MAR2020
Female
54 *
MIDAZOLAM
RESPIRATORY
DEPRESSION
THE
*
MORPHINE
SULPHATE
CARDIAC
ARREST
*
MORPHINE
SULPHATE
AGITATION
DEXAMETHASONE
136512
MAR2020
Female
67
* INFLUENZA - QUADRIVALENT
CHRONIC OBSTRUCT AIRWAYS DISEASE
UNDER
136520 MAR2020
Female
53 *
PALIPERIDONE
SUICIDE
136521
APR2020
Male
67
* INFLUENZA - QUADRIVALENT
SUDDEN DEATH
ATORVASTATIN
BENDROFLUAZIDE
FELODIPINE
ACETYLSALICYLIC
ACID
136522
APR2020
Female
71
* INFLUENZA - QUADRIVALENT
SUDDEN DEATH
ATENOLOL
RELEASED
ENALAPRIL
FRUSEMIDE
136687 MAY2020
Female
80 *
AMOXICILLIN
DRESS
SYNDROME
NEPHRITIS
INTERSTITIAL
NZPhvC – Deaths January to December 2020
3
 New Zealand Pharmacovigilance Centre
University of Otago
New Zealand Pharmacovigilance Centre
University of Otago
Email: [email address]
Website: https://nzphvc.otago.ac.nz
Report No
Date
Gender Age
Medicines/Vaccine
Reactions
136929 MAY2020
Female
70 *
CHLORAMPHENICOL
ANAEMIA
APLASTIC
PREDNISONE
137053
MAY2020
Male
71
* LENALIDOMIDE
PROGRESSION OF DISEASE
DEXAMETHASONE
1982
CYCLOPHOSPHAMIDE
137118
MAY2020
Female
54
* TRASTUZUMAB
PROGRESSION OF DISEASE
ACT
137180
JUN2020
Male
69
* PEMBROLIZUMAB
PROGRESSION OF DISEASE
*
CARBOPLATIN
MEDICINE
INEFFECTIVE
*
GEMCITABINE
137186 JUN2020
Female
79 *
ALIROCUMAB
ABDOMINAL
PAIN
ANOREXIA
BLOATING
MELAENA
137280 JUN2020
Male
58 *
CETUXIMAB
INTESTINAL
PERFORATION
ANTINEOPLASTIC AGENT(S) NOS
INFORMATION
137377 JUN2020
Female
55 *
CLOZAPINE
PNEUMONIA
137378 JUN2020
Male
55 *
CLOZAPINE
SUDDEN
DEATH
DRUG
TOXICITY
137379 JUN2020
Male
55 *
CLOZAPINE
MYOCARDIAL
INFARCTION
OFFICIAL
137380 JUN2020
Female
79 *
CLOZAPINE
ARRHYTHMIA
137381 JUN2020
Male
49 *
CLOZAPINE
THROMBOSIS
CORONARY
PANTOPRAZOLE
THE
IMIPRAMINE
AMISULPRIDE
SALBUTAMOL
137382 JUN2020
Male
47 *
CLOZAPINE
THROMBOSIS
CORONARY
UNDER
137383 JUN2020
Male
17 *
CLOZAPINE
SUDDEN
DEATH
METFORMIN
LAXSOL
ARIPIPRAZOLE
137384 JUN2020
Male
59 *
QUETIAPINE
SUICIDE
BLOOD
DISORDER
137385 JUN2020
Male
43 *
CLOZAPINE
SUDDEN
DEATH
METFORMIN
RELEASED
BENZTROPINE
MESYLATE
PROPRANOLOL
RISPERIDONE
137416
JUN2020
Female
73
* HUMAN SERUM ALBUMIN
CHILLS
PARACETAMOL
ACETYLSALICYLIC
ACID
NZPhvC – Deaths January to December 2020
4
 New Zealand Pharmacovigilance Centre
University of Otago
New Zealand Pharmacovigilance Centre
University of Otago
Email: [email address]
Website: https://nzphvc.otago.ac.nz
Report No
Date
Gender Age
Medicines/Vaccine
Reactions
137420
JUN2020
Male
20
* VELIPARIB
PROGRESSION OF DISEASE
TEMOZOLOMIDE
137451 JUN2020
Male
78 *
CLOZAPINE ASPIRATION
PNEUMONITIS
MIDODRINE
PNEUMONIA
1982
ACETYLSALICYLIC
ACID
DOCUSATE + SENNOSIDES
FLUDROCORTISONE
ACT
137452 JUN2020
Male
61 *
CLOZAPINE
MYOCARDIAL
INFARCTION
RESPIRATORY
DISORDER
137453 JUN2020
Male
76 *
CLOZAPINE
STROKE
LEVOMEPROMAZINE DEMENTIA
137454 JUN2020
Male
54 *
CLOZAPINE
CARCINOMA
MOCLOBEMIDE
ATORVASTATIN
METFORMIN
CHOLECALCIFEROL
137455 JUN2020
Male
78 *
CLOZAPINE
PNEUMONIA
INFORMATION
137486
JUN2020
Female
70
* PEMBROLIZUMAB
PROGRESSION OF DISEASE
137487
JUN2020
Male
78
* LENALIDOMIDE
PROGRESSION OF DISEASE
137495 JUN2020
Male
*
COBIMETINIB
PROGRESSION OF DISEASE
*
VEMURAFENIB
OFFICIAL
137514 JUL2020 Male
4m *
DTaP-Hexa
SUDDEN
DEATH
* PNEUMOCOCCAL VACC - 10
THE
137578
JUL2020
Female
78
* M&P VITAL ALL-IN-ONE
FEELING UNWELL
SUDDEN
DEATH
137603
JUL2020
Male
79
* HEPARIN-LMW - ENOXAPARIN
HAEMORRHAGE RETROPERITONEAL
UNDER
137611
JUL2020
Male
85
* INFLUENZA - QUADRIVALENT
UNCONSCIOUSNESS
FIXED
PUPILS
137623 JUL2020 Male
*
COBIMETINIB
CONVULSIONS
*
VEMURAFENIB
DYSPHAGIA
137668
JUL2020
Male
45
* VENLAFAXINE
THERAPEUTIC RESPONSE DECREASED
BISOPROLOL
RELEASED
137732 JUL2020 Female
85 *
RIVAROXABAN
HAEMORRHAGE
INTRACRANIAL
FALL
FRACTURE
137735 JUL2020 Male
76 *
DABIGATRAN
INTESTINAL
NECROSIS
*
IDARUCIZUMAB
NZPhvC – Deaths January to December 2020
5
 New Zealand Pharmacovigilance Centre
University of Otago
New Zealand Pharmacovigilance Centre
University of Otago
Email: [email address]
Website: https://nzphvc.otago.ac.nz
Report No
Date
Gender Age
Medicines/Vaccine
Reactions
137738 JUL2020 Female
58 *
IMMUNOGLOBULIN
NORMAL
HYPOTENSION
MORPHINE SULPHATE
BACK PAIN
*
PLATELETS
VOMITING
LAXSOL
RECTAL
BLEEDING
FAMOTIDINE
INFUSION
REACTION
1982
137761 JUL2020 Female
22 *
LAMOTRIGINE
SUDDEN
DEATH
137860 AUG2020
Male
45 *
OLANZAPINE
QT
PROLONGED
ACT
*
MORPHINE
SULPHATE FIBRILLATION
VENTRICULAR
METHAMPHETAMINE CARDIAC
ARREST
MULTIPLE ORGAN FAILURE
CONVULSIONS
137875 AUG2020
Female
62 *
CODEINE
RESPIRATORY
ARREST
CARDIAC
ARREST
INTENTIONAL
OVERDOSE
137879 AUG2020
Male
69 *
LEVODOPA/CARBIDOPA (NOS)
ASPIRATION PNEUMONITIS
LORAZEPAM
RIGORS
QUETIAPINE
MEDICATION
ERROR
GABAPENTIN
NEUTROPENIA
DOMPERIDONE
HYPERKALAEMIA
INFORMATION
137885 AUG2020
Male
63 *
PEMBROLIZUMAB
PNEUMONIA
137890
AUG2020
Male
6w
* ROTA VIRUS VACCINE
SUDDEN DEATH
*
DTaP-Hexa
* PNEUMOCOCCAL VACC - 10
CHOLECALCIFEROL
FERROUS
SULPHATE
OFFICIAL
137895 AUG2020
Female
71 *
OESTRIOL
UTERINE
CARCINOMA
THE
137934 AUG2020
Female
74 *
DABIGATRAN
PULMONARY
HAEMORRHAGE
138095 AUG2020
Female
74 *
ALECTINIB
MYALGIA
DABIGATRAN
PNEUMONITIS
FELODIPINE
PROGRESSION OF DISEASE
CHOLECALCIFEROL
SALBUTAMOL
UNDER
138123
AUG2020
Male
71
* INTRAGAM P
CONGESTIVE HEART FAILURE
DOXYCYCLINE
HYPOXIA
VALACICLOVIR
HYPOTENSION
METOPROLOL
RENAL
IMPAIRMENT
TIOTROPIUM
/OLODATEROL
HYPERKALAEMIA
138219
SEP2020
Male
6m
* DTaP-Hexa
SUDDEN INFANT DEATH SYNDROME
* PNEUMOCOCCAL VACC - 10
RELEASED
138323 SEP2020
Male
62 *
PEMBROLIZUMAB
RASH
PEMETREXED
RHINITIS
CARBOPLATIN
LACRIMATION
INCREASED
PROGRESSION OF DISEASE
NZPhvC – Deaths January to December 2020
6
 New Zealand Pharmacovigilance Centre
University of Otago
New Zealand Pharmacovigilance Centre
University of Otago
Email: [email address]
Website: https://nzphvc.otago.ac.nz
Report No
Date
Gender Age
Medicines/Vaccine
Reactions
138444
SEP2020
Female
64
*
RITUXIMAB
LEUKOENCEPHALOPATHY
*
BENDAMUSTINE
PROGRESSION
OF
DISEASE
FLUDARABINE
IMMUNE (IRIS) SYNDROME
MITOXANTRONE
PNEUMONIA
RESPIRATORY
ARREST
138645
OCT2020
Male
19
*
LAMOTRIGINE
SUDDEN
DEATH
TOPIRAMATE
MIDAZOLAM
ACT 1982
138728
OCT2020
Male
81
*
ADALIMUMAB
CARCINOMA
COLON
METASTASES
NOS
138733
OCT2020
Male
*
CHLORPROMAZINE
NEOPLASM
MALIGNANT
*
PARALDEHYDE
138843
NOV2020
Male
73
*
CLOZAPINE
PNEUMONIA
NEOPLASM
MALIGNANT
PROGRESSION OF DISEASE
138844
NOV2020
Male
70
*
CLOZAPINE
PNEUMONIA
OLANZAPINE
ATRIAL
FIBRILLATION
INFORMATION
TACHYCARDIA
VENTRICULAR
CARDIAC
ARREST
138845
NOV2020
Male
67
*
CLOZAPINE
PNEUMONIA
*
OMEPRAZOLE
SEPSIS
NICOTINE
BRONCHIECTASIS
BUDESONIDE/EFORMOTEROL
AMISULPRIDE
138846
NOV2020
Female
73
*
CLOZAPINE
PARKINSONISM
APOMORPHINE
LEVODOPA
AMANTADINE
MIRTAZAPINE
138847
NOV2020
Male
64
*
CLOZAPINE
SUDDEN
DEATH
ATENOLOL
FRUSEMIDE
LAXSOL
OMEPRAZOLE
138848
NOV2020
Male
67
*
CLOZAPINE
SUDDEN
DEATH
BENZTROPINE MESYLATE
MYOCARDIAL INFARCTION
ATORVASTATIN
138849
NOV2020
Male
91
*
CLOZAPINE ASPIRATION
PNEUMONITIS
ALENDRONATE + CHOLECALCIFEROL
CONFUSION
DONEPEZIL
SOMNOLENCE
ESCITALOPRAM
FALL
FLUDROCORTISONE
RELEASED UNDER THE OFFICIAL
138850
NOV2020
Male
52
*
CLOZAPINE
METASTASES
NOS
NEOPLASM
MALIGNANT
NEUTROPHILIA
LEUKOCYTOSIS
NZPhvC – Deaths January to December 2020
7
 New Zealand Pharmacovigilance Centre
University of Otago
New Zealand Pharmacovigilance Centre
University of Otago
Email: [email address]
Website: https://nzphvc.otago.ac.nz
Report No
Date
Gender Age
Medicines/Vaccine
Reactions
138851
NOV2020
Male
72
*
CLOZAPINE
DEMENTIA
HEAD
INJURY
138852
NOV2020
Female
72
*
CLOZAPINE
DEMENTIA
DONEPEZIL
DOCUSATE + SENNOSIDES
LORAZEPAM
VENLAFAXINE
ACT 1982
138952
NOV2020
Female
67
*
PEMBROLIZUMAB
CONVULSIONS
AGGRAVATED
139041
NOV2020
Female
80
*
CLOZAPINE
PNEUMONIA
ESCITALOPRAM
OMEPRAZOLE
FLUTICASONE/SALMETEROL
DORZOLAMIDE/TIMOLOL
139042
NOV2020
Male
71
* ATEZOLIZUMAB
PROGRESSION OF DISEASE
*
BEVACIZUMAB
139195
DEC2020
Female
* TRASTUZUMAB
PROGRESSION OF DISEASE
INFORMATION
RELEASED UNDER THE OFFICIAL
NZPhvC – Deaths January to December 2020
8
1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
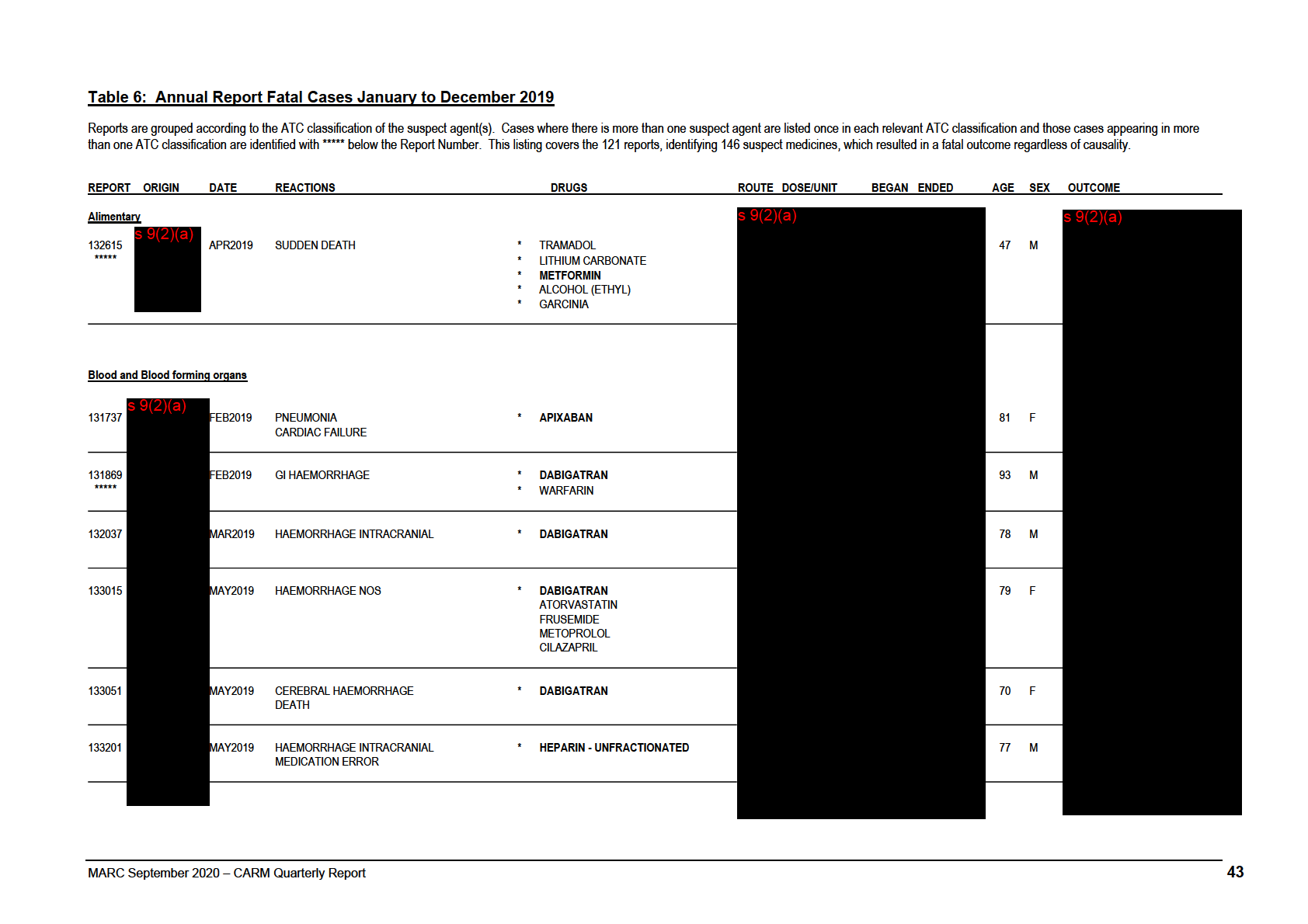
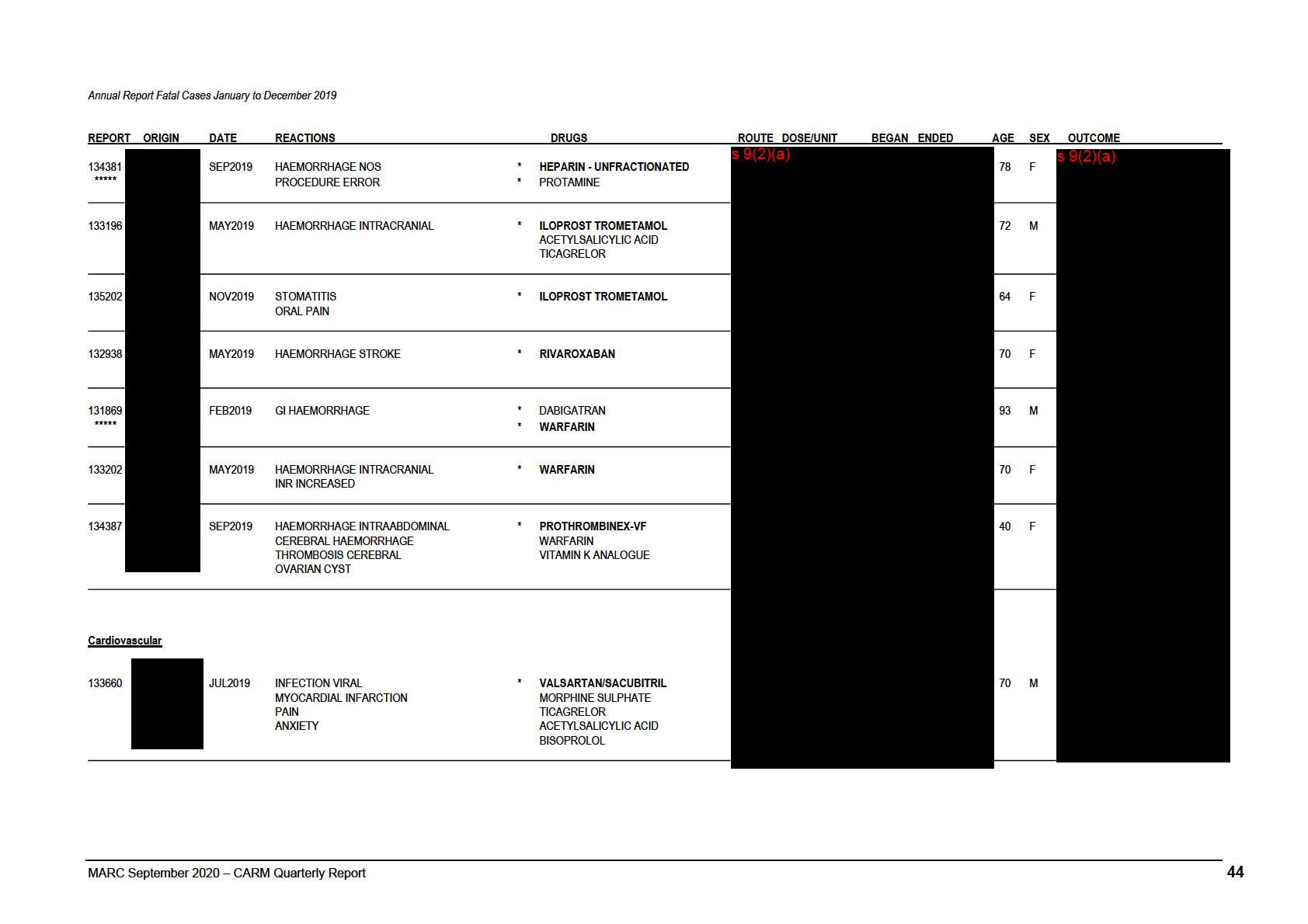
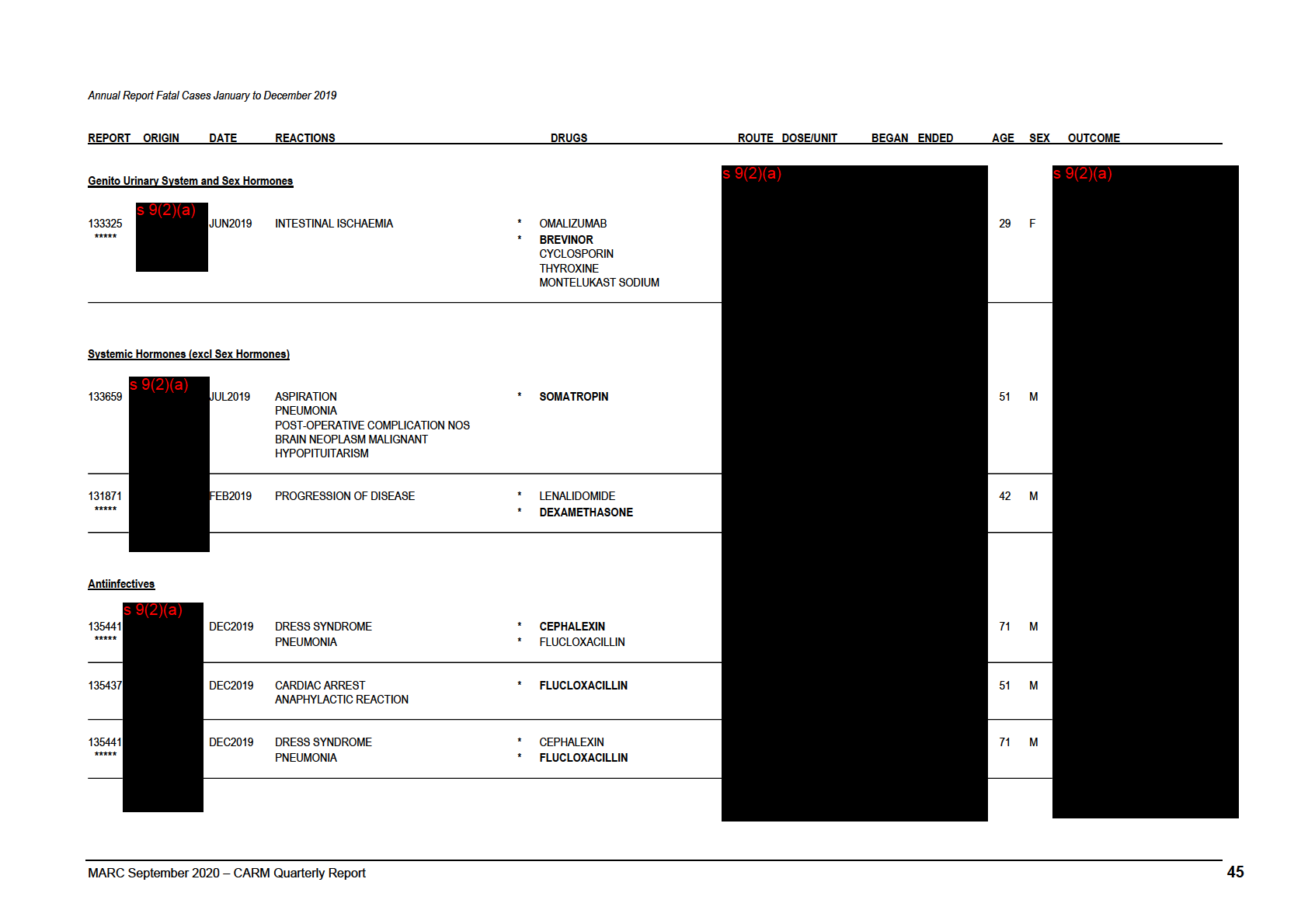
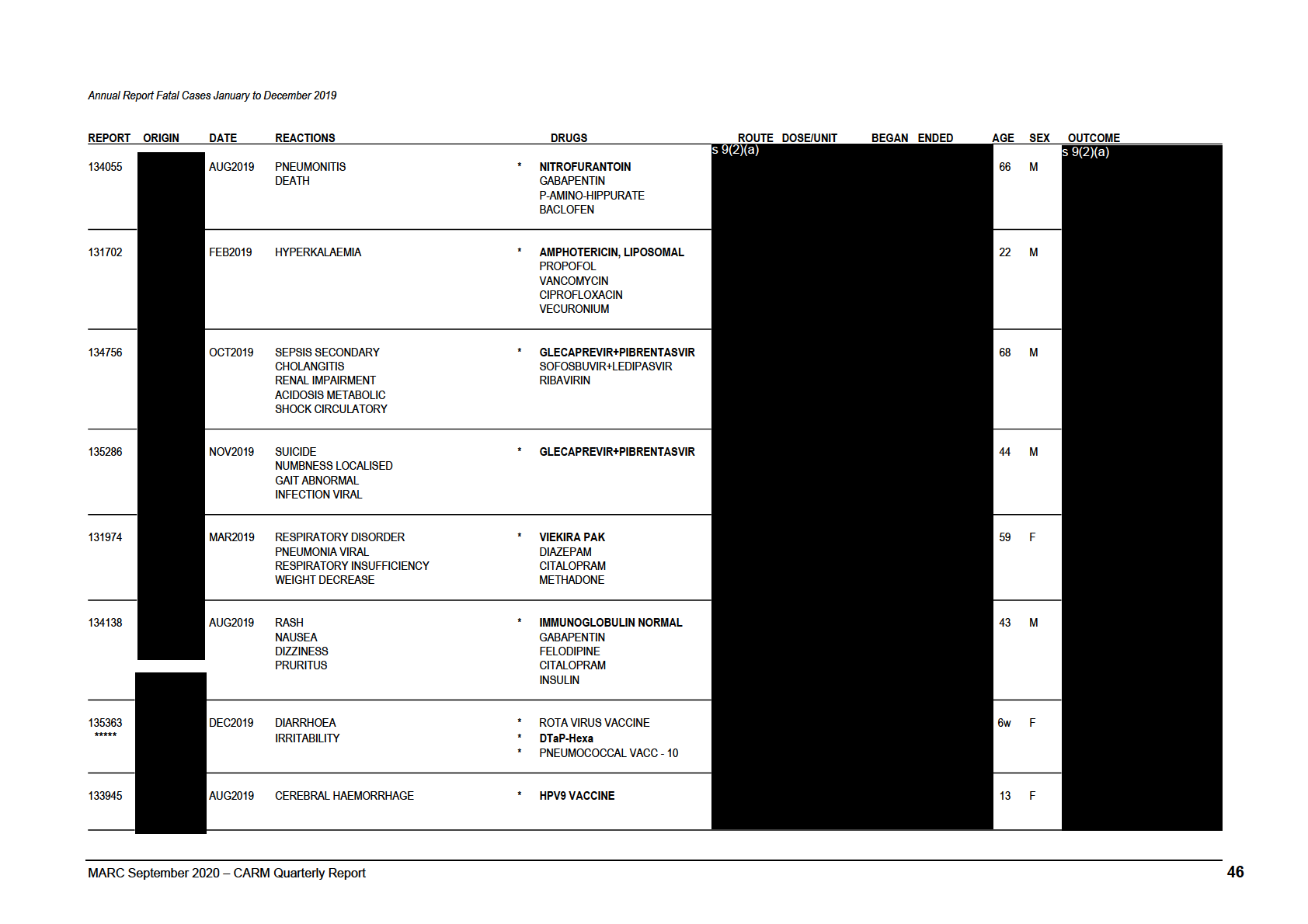
Annual Report Fatal Cases January to December 2019
REPORT ORIGIN
DATE
REACTIONS
DRUGS
ROUTE DOSE/UNIT
BEGAN ENDED
AGE SEX OUTCOME
s 9(2)(a)
s 9(2)(a)
s 9(2)(a)
133990
AUG2019 SUDDEN
DEATH
*
HPV9 VACCINE
12
F
ACT 1982
134977
NOV2019 FALL
*
HPV9 VACCINE
13
F
134643
OCT2019
SUDDEN DEATH
I
INFLUENZA - QUADRIVALENT
60
F
*****
DRUG INTERACTION
I
UNCLASSIFIED AGENT
135363
DEC2019
DIARRHOEA
*
ROTA VIRUS VACCINE
6w
F
*****
IRRITABILITY
*
DTaP-Hexa
*
PNEUMOCOCCAL VACC - 10
INFORMATION
135363
DEC2019 DIARRHOEA
*
ROTA VIRUS VACCINE
6w
F
*****
IRRITABILITY
*
DTaP-Hexa
*
PNEUMOCOCCAL VACC - 10
Antineoplastics and Immunomodulating Agents
s 9(2)(a)
s 9(2)(a)
133736 s 9(2)(a)
JUL2019 LEUKAEMIA
MYELOID
*
ADRIAMYCIN
36
F
*****
DEATH
*
BLEOMYCIN
*
VINBLASTINE
*
IFOSFAMIDE
*
CARBOPLATIN
133179
MAY2019
PROGRESSION OF DISEASE
*
AXITINIB
55
M
*****
*
PEMBROLIZUMAB
133736
JUL2019 LEUKAEMIA
MYELOID
*
ADRIAMYCIN
36
F
*****
*
BLEOMYCIN
*
VINBLASTINE
*
IFOSFAMIDE
*
CARBOPLATIN
MARC September 2020 – CARM Quarterly Report
47
RELEASED UNDER THE OFFICIAL
Annual Report Fatal Cases January to December 2019
REPORT ORIGIN
DATE
REACTIONS
DRUGS
ROUTE DOSE/UNIT
BEGAN ENDED
AGE SEX OUTCOME
s 9(2)(a)
s 9(2)(a)
133695
JUL2019
NEOPLASM MALIGNANT
*
BORTEZOMIB
89
F
s 9(2)(a)
ACT 1982
*****
*
CYCLOPHOSPHAMIDE
132015
MAR2019 HEADACHE
*
CAPECITABINE
56
M
VISION BLURRED
CARDIAC FAILURE LEFT
133736
JUL2019 LEUKAEMIA
MYELOID
*
ADRIAMYCIN
36
F
*****
DEATH
*
BLEOMYCIN
*
VINBLASTINE
*
IFOSFAMIDE
*
CARBOPLATIN
INFORMATION
131442
JAN2019
PROGRESSION OF DISEASE
*
CARFILZOMIB
73
M
CYCLOPHOSPHAMIDE
DEXAMETHASONE
135291
NOV2019
EMBOLISM PULMONARY
*
CETUXIMAB
56
F
133695
JUL2019
NEOPLASM MALIGNANT
*
BORTEZOMIB
89
F
*****
*
CYCLOPHOSPHAMIDE
134057
AUG2019
RESPIRATORY DISTRESS SYNDROME
*
CYTOSINE ARABINOSIDE
43
F
134088
AUG2019 DIARRHOEA
*
ERLOTINIB
79
F
RENAL FAILURE ACUTE
CANDESARTAN
FRUSEMIDE
134067
AUG2019 DEHYDRATION
*
FLUOROURACIL
47
M
NAUSEA
VOMITING
CARDIAC
ARREST
MARC September 2020 – CARM Quarterly Report
48
RELEASED UNDER THE OFFICIAL
Annual Report Fatal Cases January to December 2019
REPORT ORIGIN
DATE
REACTIONS
DRUGS
ROUTE DOSE/UNIT
BEGAN ENDED
AGE SEX OUTCOME
s 9(2)(a)
s 9(2)(a)
s 9(2)(a)
133736
JUL2019 LEUKAEMIA
MYELOID
*
ADRIAMYCIN
36
F
ACT 1982
*****
*
BLEOMYCIN
*
VINBLASTINE
*
IFOSFAMIDE
*
CARBOPLATIN
134082
AUG2019 INFECTION
*
NINTEDANIB
67
F
PNEUMONITIS
OMEPRAZOLE
RESPIRATORY
FAILURE
DABIGATRAN
AZITHROMYCIN
SOTALOL
131436
JAN2019 PNEUMONITIS
*
PEMBROLIZUMAB
60
M
INFORMATION
132549
APR2019 DEMYELINATION
*
PEMBROLIZUMAB
63
M
PROGRESSION OF DISEASE
132550
APR2019
PROGRESSION OF DISEASE
*
PEMBROLIZUMAB
85
M
APLASIA, PURE RED CELL
DEXAMETHASONE
THROMBOCYTOPENIA
HEPATIC ENZYMES INCREASED
133179
MAY2019
PROGRESSION OF DISEASE
*
AXITINIB
55
M
*****
*
PEMBROLIZUMAB
135292
NOV2019
PROGRESSION OF DISEASE
*
PEMBROLIZUMAB
59
F
ANTINEOPLASTIC AGENT(S) NOS
133755
JUL2019
PULMONARY FIBROSIS
*
RITUXIMAB
69
M
INTERSTITIAL LUNG DISEASE
132402
APR2019
MEDICINE INEFFECTIVE
*
VENETOCLAX
62
M
VALACICLOVIR
COTRIMOXAZOLE - BACTRIM GROUP
TRANEXAMIC ACID
MARC September 2020 – CARM Quarterly Report
49
RELEASED UNDER THE OFFICIAL
Annual Report Fatal Cases January to December 2019
REPORT ORIGIN
DATE
REACTIONS
DRUGS
ROUTE DOSE/UNIT
BEGAN ENDED
AGE SEX OUTCOME
s 9(2)(a)
133588 s 9(2)(a)
s 9(2)(a)
JUL2019
T-CELL LYMPHOMA
*
VENETOCLAX
59
M
ACT 1982
HISTIOCYTOSIS HAEMATOPHAGIC (HLH)
MULTIVITAMINS
133736
JUL2019 LEUKAEMIA
MYELOID
*
ADRIAMYCIN
36
F
*****
*
BLEOMYCIN
*
VINBLASTINE
*
IFOSFAMIDE
*
CARBOPLATIN
134016
AUG2019
NEOPLASM MALIGNANT
*
ADALIMUMAB
56
M
135472
DEC2019 STROKE
*
ADALIMUMAB
72
M
INFORMATION
PACKAGING CHANGE
ISONIAZID
PYRIDOXINE
METFORMIN
CILAZAPRIL
133532
JUN2019
THROMBOSIS VENOUS DEEP
*
INFLIXIMAB
74
F
FRACTURE
CILAZAPRIL
PNEUMONIA
TAMOXIFEN
RESPIRATORY
FAILURE
MESALAZINE
RENAL FAILURE CHRONIC
AZATHIOPRINE
134058
AUG2019 PANCYTOPENIA
*
LEFLUNOMIDE
59
F
MULTIPLE ORGAN FAILURE
MACROPHAGE ACTIVATION SYNDROME
131871
FEB2019
PROGRESSION OF DISEASE
*
LENALIDOMIDE
42
M
*****
*
DEXAMETHASONE
131979
MAR2019
CARCINOMA SQUAMOUS
*
LENALIDOMIDE
76
M
132941
MAY2019
MEDICINE INEFFECTIVE
*
LENALIDOMIDE
66
F
PROGRESSION OF DISEASE
MARC September 2020 – CARM Quarterly Report
50
RELEASED UNDER THE OFFICIAL
Annual Report Fatal Cases January to December 2019
REPORT ORIGIN
DATE
REACTIONS
DRUGS
ROUTE DOSE/UNIT
BEGAN ENDED
AGE SEX OUTCOME
s 9(2)(a)
s 9(2)(a)
132942
MAY2019
PROGRESSION OF DISEASE
*
LENALIDOMIDE
80
M
ACT 1982
131621
JAN2019
PROGRESSION OF DISEASE
*
POMALIDOMIDE
72
F
131872
FEB2019
MYELOMATOSIS MULTIPLE
*
POMALIDOMIDE
77
M
131873
FEB2019
PROGRESSION OF DISEASE
*
POMALIDOMIDE
80
F
DEXAMETHASONE
SEX
INFORMATION
133887
JUL2019
PROGRESSION OF DISEASE
*
POMALIDOMIDE
82
M
134133
AUG2019
PROGRESSION OF DISEASE
*
POMALIDOMIDE
72
F
Musculoskeletal
133270
JUN2019
GASTRIC ULCER PERFORATED
*
DICLOFENAC
62
M
RENAL FAILURE ACUTE
*
DICLOFENAC
PERITONITIS
*
DICLOFENAC
MULTIPLE ORGAN FAILURE
133747
JUL2019 CONFUSION
*
GABAPENTIN
57
M
*****
CONSCIOUSNESS
DECREASED
*
BACLOFEN
MEDICATION
ERROR
132344
MAR2019
MEDICATION ERROR
*
BOTULINUM TOXIN
63
F
DYSPHAGIA
FELODIPINE
GABAPENTIN
BACLOFEN
THYROXINE
MARC September 2020 – CARM Quarterly Report
51
RELEASED UNDER THE OFFICIAL
Annual Report Fatal Cases January to December 2019
REPORT ORIGIN
DATE
REACTIONS
DRUGS
ROUTE DOSE/UNIT
BEGAN ENDED
AGE SEX OUTCOME
ACT 1982
Nervous System
s 9(2)(a)
s 9(2)(a)
s 9(2)(a)
134431
SEP2019 SUICIDE
*
SUMATRIPTAN
58
F
THERAPEUTIC RESPONSE DECREASED
132615
APR2019 SUDDEN
DEATH
*
TRAMADOL
47
M
*****
*
LITHIUM CARBONATE
*
METFORMIN
*
ALCOHOL (ETHYL)
*
GARCINIA
133747
JUL2019 CONFUSION
*
GABAPENTIN
57
M
INFORMATION
*****
CONSCIOUSNESS
DECREASED
*
BACLOFEN
MEDICATION
ERROR
134668
OCT2019 SUDDEN
DEATH
*
LAMOTRIGINE
23
F
HEADACHE
FEELING
UNWELL
HEAD
INJURY
134847
OCT2019 SUDDEN
DEATH
*
LAMOTRIGINE
24
M
VALPROATE SODIUM
ACETYLSALICYLIC ACID
Cannabis
134878
OCT2019 SUDDEN
DEATH
*
LAMOTRIGINE
27
M
135078
NOV2019 SUDDEN
DEATH
*
LAMOTRIGINE
26
M
*****
CONVULSIONS
*
CLOBAZAM
135571
DEC2019
THERAPEUTIC RESPONSE DECREASED
*
LAMOTRIGINE
31
M
CARDIAC ARREST
VOMITING
ASPIRATION
AURA
MARC September 2020 – CARM Quarterly Report
52
RELEASED UNDER THE OFFICIAL
Annual Report Fatal Cases January to December 2019
1982
REPORT ORIGIN
DATE
REACTIONS
DRUGS
ROUTE DOSE/UNIT
BEGAN ENDED
AGE SEX OUTCOME
s 9(2)(a)
135621
DEC2019 THROMBOCYTOPENIA
* VALPROATE
SODIUM
s 9(2)(a)
s 9(2)(a)
58
M
ACT
*****
NEUTROPENIA
*
LEVETIRACETAM
SUBDURAL
HAEMATOMA
OMEPRAZOLE
135621
DEC2019 THROMBOCYTOPENIA
*
VALPROATE SODIUM
58
M
*****
NEUTROPENIA
*
LEVETIRACETAM
SUBDURAL
HAEMATOMA
OMEPRAZOLE
132417
APR2019
PROGRESSION OF DISEASE
*
ROTIGOTINE
80
F
PNEUMONIA
LEVODOPA
INFECTION
VIRAL
133382
JUN2019 CARDIOMYOPATHY
* CLOZAPINE
44
F
INFORMATION
*****
CARDIAC
ARREST
*
AMISULPRIDE
135078
NOV2019
SUDDEN DEATH
*
LAMOTRIGINE
26
M
*****
CONVULSIONS
*
CLOBAZAM
131769
FEB2019 PNEUMONIA
*
CLOZAPINE
69
M
OFFICIAL
131770
FEB2019 PNEUMONIA
*
CLOZAPINE
75
M
THE
131771
FEB2019 PNEUMOTHORAX
*
CLOZAPINE
64
M
LOWER RESP TRACT INFECTION
DILTIAZEM
MEGACOLON
ACQUIRED
LAXSOL
ATRIAL
FIBRILLATION
OMEPRAZOLE
MEDICATION
ERROR
CLONAZEPAM
131772
FEB2019 PARKINSONISM
*
CLOZAPINE
69
F
UNDER
MARC September 2020 – CARM Quarterly Report
53
RELEASED
Annual Report Fatal Cases January to December 2019
1982
REPORT ORIGIN
DATE
REACTIONS
DRUGS
ROUTE DOSE/UNIT
BEGAN ENDED
AGE SEX OUTCOME
s 9(2)(a)
s 9(2)(a)
s 9(2)(a)
131785
FEB2019 NEUTROPHILIA
*
CLOZAPINE
52
M
ACT
LEUKOCYTOSIS
RENAL FAILURE ACUTE
URINARY TRACT INFECTION
URINARY
INCONTINENCE
131786
FEB2019 DIABETES INSIPIDUS NEPHROGENIC
*
CLOZAPINE
82
M
*****
RENAL FAILURE ACUTE
*
LITHIUM CARBONATE
NEUTROPHILIA
LEUKOCYTOSIS
PNEUMONIA
131787
FEB2019
ARRHYTHMIA VENTRICULAR
*
CLOZAPINE
77
F
INFORMATION
131788
FEB2019
BRAIN METASTASES
*
CLOZAPINE
55
F
131789
FEB2019
NEOPLASM MALIGNANT
*
CLOZAPINE
57
F
BRAIN METASTASES
OFFICIAL
131790
FEB2019 ASPIRATION PNEUMONITIS
*
CLOZAPINE
79
M
131791
FEB2019 ASPIRATION PNEUMONITIS
*
CLOZAPINE
73
M
THE
GI HAEMORRHAGE
NEUTROPHILIA
131792
FEB2019 THROMBOEMBOLISM
*
CLOZAPINE
95
F
MYOCARDIAL ISCHAEMIA
DEMENTIA
BIPOLAR AFFECTIVE DISORDER
FRACTURE
UNDER
131793
FEB2019
INTESTINAL OBSTRUCTION
*
CLOZAPINE
76
F
SEPTICAEMIA
OLANZAPINE
URINARY TRACT INFECTION
CIPROFLOXACIN
RENAL FAILURE CHRONIC
METRONIDAZOLE
CHRONIC
OBSTRUCT
AIRWAYS
DISEASE
MARC September 2020 – CARM Quarterly Report
54
RELEASED
Annual Report Fatal Cases January to December 2019
1982
REPORT ORIGIN
DATE
REACTIONS
DRUGS
ROUTE DOSE/UNIT
BEGAN ENDED
AGE SEX OUTCOME
131794 s 9(2)(a)
s 9(2)(a)
s 9(2)(a)
FEB2019 PNEUMONIA
*
CLOZAPINE
72
F
ACT
PULMONARY CARCINOMA
131795
FEB2019 OSTEOMYELITIS
*
CLOZAPINE
65
M
SEPSIS
MORPHINE
SULPHATE
PERIPHERAL
ISCHAEMIA
LORAZEPAM
VENLAFAXINE
MIRTAZAPINE
131796
FEB2019
PULMONARY CARCINOMA
*
CLOZAPINE
60
M
METASTASES NOS
CHRONIC OBSTRUCT AIRWAYS DISEASE
INFORMATION
131797
FEB2019
CARDIAC ARREST
*
CLOZAPINE
73
M
VALPROATE SODIUM
FLUDROCORTISONE
LAXSOL
FRUSEMIDE
131798
FEB2019
INTESTINAL OBSTRUCTION
*
CLOZAPINE
73
F
PNEUMONIA
LAXSOL
FRUSEMIDE
PANTOPRAZOLE
OFFICIAL
BUMETANIDE
131799
FEB2019
PULMONARY CARCINOMA
*
CLOZAPINE
67
M
BONE METASTASES
PREDNISONE
THE
PLEURAL EFFUSION
AMOXICILLIN/CLAVULANIC ACID
MELANOMA
MALIGNANT
ZOPICLONE
LYMPHANGITIS
MORPHINE
SULPHATE
131800
FEB2019 DEMENTIA
*
CLOZAPINE
59
F
VOMITING
LAXSOL
SYNCOPE
OMEPRAZOLE
UNDER
CONFUSION
AGGRAVATED
MIDAZOLAM
MORPHINE SULPHATE
MARC September 2020 – CARM Quarterly Report
55
RELEASED
Annual Report Fatal Cases January to December 2019
1982
REPORT ORIGIN
DATE
REACTIONS
DRUGS
ROUTE DOSE/UNIT
BEGAN ENDED
AGE SEX OUTCOME
s 9(2)(a)
s 9(2)(a)
s 9(2)(a)
133097
MAY2019
METASTASES NOS
*
CLOZAPINE
57
M
ACT
LEUKOCYTOSIS
AMISULPRIDE
NEUTROPENIA
PAROXETINE
NEUTROPHILIA
MOLAXOLE
CONSTIPATION
OMEPRAZOLE
133382
JUN2019 CARDIOMYOPATHY
*
CLOZAPINE
44
F
*****
CARDIAC ARREST
*
AMISULPRIDE
133383
JUN2019 LEUKOCYTOSIS
*
CLOZAPINE
64
M
NEUTROPHILIA
133888
JUL2019
NEOPLASM MALIGNANT
*
CLOZAPINE
64
F
INFORMATION
133889
JUL2019 ASPIRATION PNEUMONITIS
*
CLOZAPINE
84
F
PSYCHOSIS AGGRAVATED
LAMOTRIGINE
THYROXINE
CHOLECALCIFEROL
LAXSOL
OFFICIAL
133890
JUL2019 ASPIRATION PNEUMONITIS
*
CLOZAPINE
93
F
THYROXINE
LAXSOL
THE
133891
JUL2019
CARDIOVASCULAR DISORDERS
*
CLOZAPINE
70
F
GASTRO-INTESTINAL DISORDER NOS
CHRONIC OBSTRUCT AIRWAYS DISEASE
133892
JUL2019 PARKINSONISM
*
CLOZAPINE
69
M
CHRONIC OBSTRUCT AIRWAYS DISEASE
UNDER
133893
JUL2019
PULMONARY CARCINOMA
*
CLOZAPINE
64
M
METASTASES NOS
MARC September 2020 – CARM Quarterly Report
56
RELEASED
Annual Report Fatal Cases January to December 2019
1982
REPORT ORIGIN
DATE
REACTIONS
DRUGS
ROUTE DOSE/UNIT
BEGAN ENDED
AGE SEX OUTCOME
s 9(2)(a)
s 9(2)(a)
s 9(2)(a)
133894
JUL2019 PNEUMONIA
*
CLOZAPINE
82
M
ACT
NEUTROPHILIA
MADOPAR
PARKINSONISM
METOPROLOL
MYOCARDIAL ISCHAEMIA
LEVODOPA/CARBIDOPA (100/25)
QUETIAPINE
133895
JUL2019 PNEUMONIA
*
CLOZAPINE
74
M
PARKINSONISM
ther
133896
JUL2019
RESPIRATORY FAILURE
*
CLOZAPINE
90
M
LOWER RESP TRACT INFECTION
PARKINSONISM
INFORMATION
133897
JUL2019 PNEUMONIA
*
CLOZAPINE
74
M
SEPTIC
ARTHRITIS
SELEGILINE
LEVODOPA/CARBIDOPA (100/25)
DONEPEZIL
FLUCLOXACILLIN
133898
JUL2019 PNEUMONIA
*
CLOZAPINE
87
F
OFFICIAL
133899
JUL2019
MYOCARDIAL INFARCTION
*
CLOZAPINE
68
F
CORONARY ARTERY DISORDER
133971
AUG2019 NEUTROPHILIA
*
CLOZAPINE
38
M
THE
LEUKOCYTOSIS
134815
OCT2019
BLADDER CARCINOMA
*
CLOZAPINE
61
F
ASPIRATION
PNEUMONITIS
134816
OCT2019 PNEUMONIA
*
CLOZAPINE
58
M
UNDER
INTESTINAL OBSTRUCTION
MULTIPLE ORGAN FAILURE
INFLUENZA-LIKE
SYMPTOMS
MARC September 2020 – CARM Quarterly Report
57
RELEASED
Annual Report Fatal Cases January to December 2019
1982
REPORT ORIGIN
DATE
REACTIONS
DRUGS
ROUTE DOSE/UNIT
BEGAN ENDED
AGE SEX OUTCOME
s 9(2)(a)
s 9(2)(a)
s 9(2)(a)
134817
OCT2019
CARCINOMA COLON
*
CLOZAPINE
51
M
ACT
134818
OCT2019
RESPIRATORY FAILURE
*
CLOZAPINE
50
M
CHRONIC OBSTRUCT AIRWAYS DISEASE
BUDESONIDE/EFORMOTEROL
CARDIAC
FAILURE
AMISULPRIDE
HYPOVENTILATION
TIOTROPIUM
BROMIDE
OBESITY
MOLAXOLE
134819
OCT2019 SEPSIS
*
CLOZAPINE
67
F
ASPIRATION
PNEUMONITIS
AORTIC
STENOSIS
INFORMATION
134820
OCT2019
RESPIRATORY FAILURE
*
CLOZAPINE
65
M
CHRONIC OBSTRUCT AIRWAYS DISEASE
TIOTROPIUM /OLODATEROL
PNEUMONIA
FLUTICASONE
CLONAZEPAM
METFORMIN
134821
OCT2019
MYOCARDIAL INFARCTION
*
CLOZAPINE
65
M
OFFICIAL
134822
OCT2019 PNEUMONIA
*
CLOZAPINE
76
F
LORAZEPAM
TRAMADOL
TEMAZEPAM
LITHIUM CARBONATE
THE
134823
OCT2019
RESPIRATORY ARREST
*
CLOZAPINE
50
M
COR PULMONALE
AMISULPRIDE
CHRONIC OBSTRUCT AIRWAYS DISEASE
ATORVASTATIN
OBESITY
DOCUSATE + SENNOSIDES
CONSTIPATION
INSULIN
ASPART
UNDER
134824
OCT2019
MYOCARDIAL INFARCTION
*
CLOZAPINE
70
M
RESPIRATORY ARREST
MARC September 2020 – CARM Quarterly Report
58
RELEASED
Annual Report Fatal Cases January to December 2019
1982
REPORT ORIGIN
DATE
REACTIONS
DRUGS
ROUTE DOSE/UNIT
BEGAN ENDED
AGE SEX OUTCOME
131786
s 9(2)(a)
s 9(2)(a)
s 9(2)(a)
FEB2019
DIABETES INSIPIDUS NEPHROGENIC *
CLOZAPINE
82
M
ACT
*****
RENAL FAILURE ACUTE
*
LITHIUM CARBONATE
NEUTROPHILIA
LEUKOCYTOSIS
PNEUMONIA
132615
APR2019
SUDDEN DEATH
*
TRAMADOL
47
M
*****
*
LITHIUM CARBONATE
*
METFORMIN
*
ALCOHOL (ETHYL)
*
GARCINIA
134109
AUG2019
LITHIUM TOXICITY
*
LITHIUM CARBONATE
60
F
ENCEPHALOPATHY
INFORMATION
ACIDOSIS
DIABETES INSIPIDUS NEPHROGENIC
131741
FEB2019 ARRHYTHMIA
*
PALIPERIDONE
M
132131
MAR2019 SUICIDE
*
PALIPERIDONE
26
M
OFFICIAL
Respiratory
THE
s 9(2)(a)
132411
APR2019
CHEST PAIN
*
MEPOLIZUMAB
54
M
SUDDEN DEATH
ATORVASTATIN
MONTELUKAST SODIUM
UMECLIDINIUM BROMIDE
PREDNISONE
UNDER
133325
JUN2019
INTESTINAL ISCHAEMIA
*
OMALIZUMAB
29
F
*****
*
BREVINOR
CYCLOSPORIN
THYROXINE
MONTELUKAST SODIUM
MARC September 2020 – CARM Quarterly Report
59
RELEASED
Annual Report Fatal Cases January to December 2019
1982
REPORT ORIGIN
DATE
REACTIONS
DRUGS
ROUTE DOSE/UNIT
BEGAN ENDED
AGE SEX OUTCOME
ACT
Miscellaneous
s 9(2)(a)
s 9(2)(a)
s 9(2)(a)
132615
APR2019
SUDDEN DEATH
*
TRAMADOL
47
M
*****
*
LITHIUM CARBONATE
*
METFORMIN
*
ALCOHOL (ETHYL)
*
GARCINIA
135203
NOV2019 MELAENA
*
IDARUCIZUMAB
70
F
INTESTINAL ISCHAEMIA
METRONIDAZOLE
DABIGATRAN
INFORMATION
134381
SEP2019
HAEMORRHAGE NOS
*
HEPARIN - UNFRACTIONATED
78
F
*****
PROCEDURE
ERROR
*
PROTAMINE
132615
APR2019
SUDDEN DEATH
*
TRAMADOL
47
M
*****
*
LITHIUM CARBONATE
*
METFORMIN
*
ALCOHOL (ETHYL)
*
GARCINIA
OFFICIAL
134643
OCT2019
SUDDEN DEATH
I
INFLUENZA - QUADRIVALENT
60
F
*****
DRUG
INTERACTION
I
UNCLASSIFIED AGENT
THE
UNDER
MARC September 2020 – CARM Quarterly Report
60
RELEASED














