 MINUTES: COVID-19 Vaccine Technical Advisory Group
Date:
MINUTES: COVID-19 Vaccine Technical Advisory Group
Date:
Tuesday 3 August 2021
Time:
11:00am to 12:00pm
Location:
Teams: 9(2)(k)
Chair:
Ian Town
David Murdoch, Ian Frazer, James Ussher, Jono Hoogerbrug, Nikki Moreland,
Members:
Nikki Turner, Peter McIntyre, Sue Crengle, Tony Walls
Andi Shirtcliffe, Brooke Hollingshead, Daniel Bernal, Edwin Reynolds, Fiona
Ministry of Health Attendees:
ACT 1982
Callaghan, Shayma Faircloth,
Guests:
Christian Marchello, Kris Golding
Caroline McElnay, Elizabeth Wilson, Helen Petousis-Harris, Juliet Rumball-
Apologies:
Smith, Niki Stefanogiannis, Sean Hanna
INFORMATION
Welcome and previous minutes
1.0
Ian Town welcomed all Members, Attendees and Guests in his capacity as Chair of the COVID-19
Vaccine Technical Advisory Group (CV TAG).
Minutes of the last meeting (27 July 2021) were accepted.
Science Updates
2.0
The Chair advised that in future, the science documents on the vaccines and research in children
will be moved to the back of the materials as an appendix. The documents will be updated for the
fortnightly meetings, however the agenda will include a discussion prompt once a month.
Research in children
3.0
Item covered under 2.0, and the same discussion prompt will apply for future meetings.
Myocarditis Recommendations Update
4.0
The Chair updated CV TAG on progress with the final recommendations on myocarditis.
•
RELEASED UNDER THE OFFICIAL
The Director-General has accepted the recommendations. An announcement and
implementation plan for extending the dosing interval is forthcoming.
•
It will result in significant programmatic changes and has important equity considerations,
however the emphasis on distributing first doses to priority groups has been noted and
accepted.
Decision to Use Pfizer 12- to 15-year-olds and Children Priority Groups
5.0
•
The challenges posed by the Delta variant and emerging data on differences in clinical
severity among children were discussed with respect to vaccination in children.
• Earlier advice had been that a broader decision on vaccinating 12- to 15-year-olds should be
deferred.
• Aotearoa New Zealand’s lack of community transmission was noted as an important
consideration in making this decision.
• An exception should be made for priority groups of 12- to 15-year-olds that are at higher risk
from COVID-19 due to prior comorbidities, as are outlined in the draft memo, which CV TAG
supported.
• Vaccinations as part of outbreak management, for example in schools, was also considered
an exception.
• Opportunities provided by mass vaccination events and vaccinating whānau together were
noted as important considerations.
• The Decision to Use for 12 to 15-year-olds and memo on priority groups will be provided to
1982
the Director-General and the COVID-19 Vaccine and Immunisation Programme (CVIP).
Dosing interval for Pfizer
6.0
ACT
• The Request for Advice (RfA) on this topic was reviewed.
• The data on improved immune responses with a delayed interval was noted as promising.
• It was noted that, in the event of an outbreak, there would be reduced protection for those
who have only had one dose. CV-TAG therefore encouraged surge capacity to be built into
the programme in case of an outbreak.
• Exceptions to the longer intervals among immunosuppressed people (e.g., with solid
INFORMATION
tumours) was discussed, and the Science and Technical Advisory team will progress
consultation and discussion on these exceptions.
• The RfA on evidence on the dosing intervals will be shared with the Director-General.
Future Vaccine Portfolio
7.0
OFFICIAL
• The Ministry’s Policy team has requested CV TAG advice on considerations for ongoing
purchasing for New Zealand’s vaccine portfolio from a scientific perspective.
THE
• The RfA prepared by the Science and Technical Advisory Team on this topic was reviewed.
Data on immunity, ’booster’ doses, safety concerns and the impact of variants was
discussed.
UNDER
• Data on long-term immunogenicity and antibody levels are still emerging, however initial
data suggests immunity is long-lasting (at least 8 months for antibody levels). Currently
there are no precise correlates of protection, however the presence of neutralising
antibodies is a useful measure.
• Further evidence on immunogenicity and clinical outcomes are awaited.
• It was n
RELEASED oted that there may be other factors impacting purchasing outside of scientific or
clinical evidence, and that some countries have begun purchasing booster doses.
• Local immunogenicity data needs to be incorporated into the Request for Advice, and it was
noted that VAANZ would be collecting some further local information in their clinical trial
currently underway.
• Within the wider portfolio, it was noted that a formal application had not yet been received by
Medsafe from Novavax.
• Evidence regarding heterologous vaccine schedules is emerging, and will be a consideration
for those individuals who require an alternative to Pfizer.
 1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
 1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
 MINUTES: COVID-19 Vaccine Technical Advisory Group
Date:
MINUTES: COVID-19 Vaccine Technical Advisory Group
Date:
Tuesday 17 August 2021
Time:
11:00am to 12:00pm
Location:
Teams: 9(2)(k)
Chair:
Ian Town
Elizabeth Wilson, Helen Petousis-Harris, James Ussher, Nikki Moreland, Nikki
1982
Members:
Turner, Peter McIntyre, Sean Hanna, Sue Crengle, Tony Walls
Andi Shirtcliffe, Brooke Hollingshead, Daniel Bernal, Edwin Reynolds, Fiona
ACT
Ministry of Health Attendees:
Callaghan, Juliet Rumball-Smith, Niki Stefanogiannis, Shayma Faircloth, Pippa
Scott
Guests:
Christian Marchello, John Tait, Kris Golding, Rachel Eyre, Tia Narvaez
Apologies:
Caroline McElnay, David Murdoch, Ian Frazer
INFORMATION
Welcome and previous minutes
1.0
Ian Town welcomed all Members and Attendees in his capacity as Chair of the COVID-19 Vaccine
Technical Advisory Group (CV TAG). Mr John Tait, Chair of the Vaccine ISMB was welcomed.
Minutes of the last meeting (03 August 2021) were accepted.
OFFICIAL
Science Updates
2.0
THE
Updates on the COVID-19 vaccines were highlighted:
• There are reports that the US intends to grant full approval of Pfizer in early September.
• The US Food and Drug Administration have amended their emergency use authorisations
UNDER
for Pfizer and Moderna to allow for the use of a third dose in certain immunocompromised
people, specifically, solid organ transplant recipients or those who are diagnosed with
conditions that are considered to have an equivalent level of immunocompromise.
• Pfizer have released preliminary data on third doses in adults showing increased
neutralising antibody titres.
•
RELEASED
More data on the safety of vaccination among pregnant women has been released.
Research in children
3.0
Updates on COVID-19 vaccine research among children were highlighted:
• CV TAG requested information on whether any post-marketing larger summaries have been
released relating to children. Some initial data from the CDC were shared, and this will be
included in future updates.
Vaccine Rollout Update
4.0
The daily vaccine report was presented to CV TAG. The rollout is proceeding at pace and ramping
up to deliver 50,000+ doses per day. Supplies are now steady. The 2020 Health Service Utilisation
is being used as the population denominator in order to monitor vaccination data by ethnicity.
Dosing Interval for Pfizer
5.0
The Chair shared that the extension of the interval between doses was accepted by the Director-
General and announced by the Prime Minister last week and was framed as providing greater
population protection. The changes to the booking website have been implemented and this has
freed up appointments for more first doses around New Zealand.
Myocarditis after Pfizer Vaccination
6.0
An update on myocarditis cases was provided by the Chair:
• The risk management communication relating to myocarditis was addressed with the
announcement of the dosing interval extension. It was requested that references to
1982
increasing dosing intervals potentially providing some protection against myocarditis be
removed from communications. This has been actioned.
ACT
• An amendment to CV TAG’s recommendations on myocarditis after Pfizer COVID-19
vaccination is needed to confirm that those “under clinical review by a cardiologist who
should discuss the risk and benefits of vaccination” applies for 12- to 29-year-olds (and not
16- to 29-year-olds) once the extended age range has been approved and announced.
Decision to Use Pfizer 12- to 15-year-olds
7.0
• CV TAG’s recommendation that vaccination of 12- to 15-year-olds proceeds has been
relayed to the Director-General and Vaccine Ministers.
INFORMATION
• Advice on promoting vaccination in whānau groups has been incorporated.
• CV TAG requested the benefits of personal and family protection should be emphasised,
rather than indirect benefits such as population protection.
•
OFFICIAL
The importance of vaccinating vulnerable groups among 12- to 15-year-olds was raised and
discussed. It was noted that 12- to 15-year-olds considered Group 3 will be prioritised
through another pathway and given codes to book.
THE
MMR/Influenza Coadministration
8.0
A draft memo reviewing evidence on coadministration of the COVID-19 vaccine with other vaccines
(e.g. MMR/Influenza/HPV) was shared with CV TAG for discussion.
UNDER
• CV TAG discussed the immunisation programme in the context of concern about RSV
outbreaks and impact on staffing, lagging vaccination rates for MMR and HPV, and
knowledge of the prior impact of measles outbreaks on Māori and Pasifika.
• CV TAG encouraged that all intervals between COVID-19 vaccines and other vaccines (with
the exception below) be removed, and same-day coadministration be allowed. Such
RELEASED
intervals were seen as a barrier to uptake of both the COVID-19 vaccine and other
vaccinations.
• An exception to same-day coadministration should be made for the live-attenuated shingles
vaccine (Zostavax), where a 7-day interval is still required.
• It was noted that younger people produce a good immune response to the COVID-19
vaccine and therefore even if this immune response is reduced by coadministration, it would
still likely provide excellent protection.
• The science on coadministration will continue to be monitored by the Science and Technical
Advisory team.
• The advice memo will be updated to reflect this messaging and shared with CVIP.
Other COVID-19 Vaccines that New Zealand Could Recognise for Border Workers
9.0
• The Ministry’s Policy team have requested CV TAG’s advice on which other vaccines (in
addition to Pfizer) should be recognised among border workers, and how to approach
incomplete vaccinations among border workers.
• Medsafe has advised that the Ministry should adopt vaccines provisionally approved or
authorised through emergency use provisions by:
1. Medsafe themselves and
2. Regulators in countries with similar regulatory systems to New Zealand, including
the Australian Therapeutic Goods Administration, the US Food and Drug
Administration, Health Products and Food Branch of Health Canada, United
Kingdom Medicines and Healthcare products Regulatory Agency, and the European
1982
Medicines Agency.
• The current advice is that if someone is partially vaccinated and only has a single-dose of a
ACT
two-dose course regimen from overseas, they should have a dose of Pfizer after at least
four weeks. It was noted that there should be no upper limit on when the second dose can
be administered, and courses did not have to be repeated if there had been a long interval.
They also advised against the use of serology/antibody testing to check protection.
• CV TAG noted that any guidance to border workers could potentially apply more generally to
all overseas arrivals.
• Sinopharm and Sinovac vaccines were discussed, with mention of recipients of these
INFORMATION
possibly needing a booster dose of Pfizer to provide sufficient protection. However, it was
noted that a complete review of the evidence on protection offered by other vaccines and
incomplete vaccination schedules is needed to inform the discussion.
• The Science and Technical Advisory team will conduct a review of the evidence and share
this with CV TAG for discussion at a future meeting.
OFFICIAL
Next Steps/Decisions Pending
10.0
None.
THE
Any Other Business
11.0
None.
UNDER
Agenda items for next meeting
12.0
None.
New Action Items Raised During Meeting
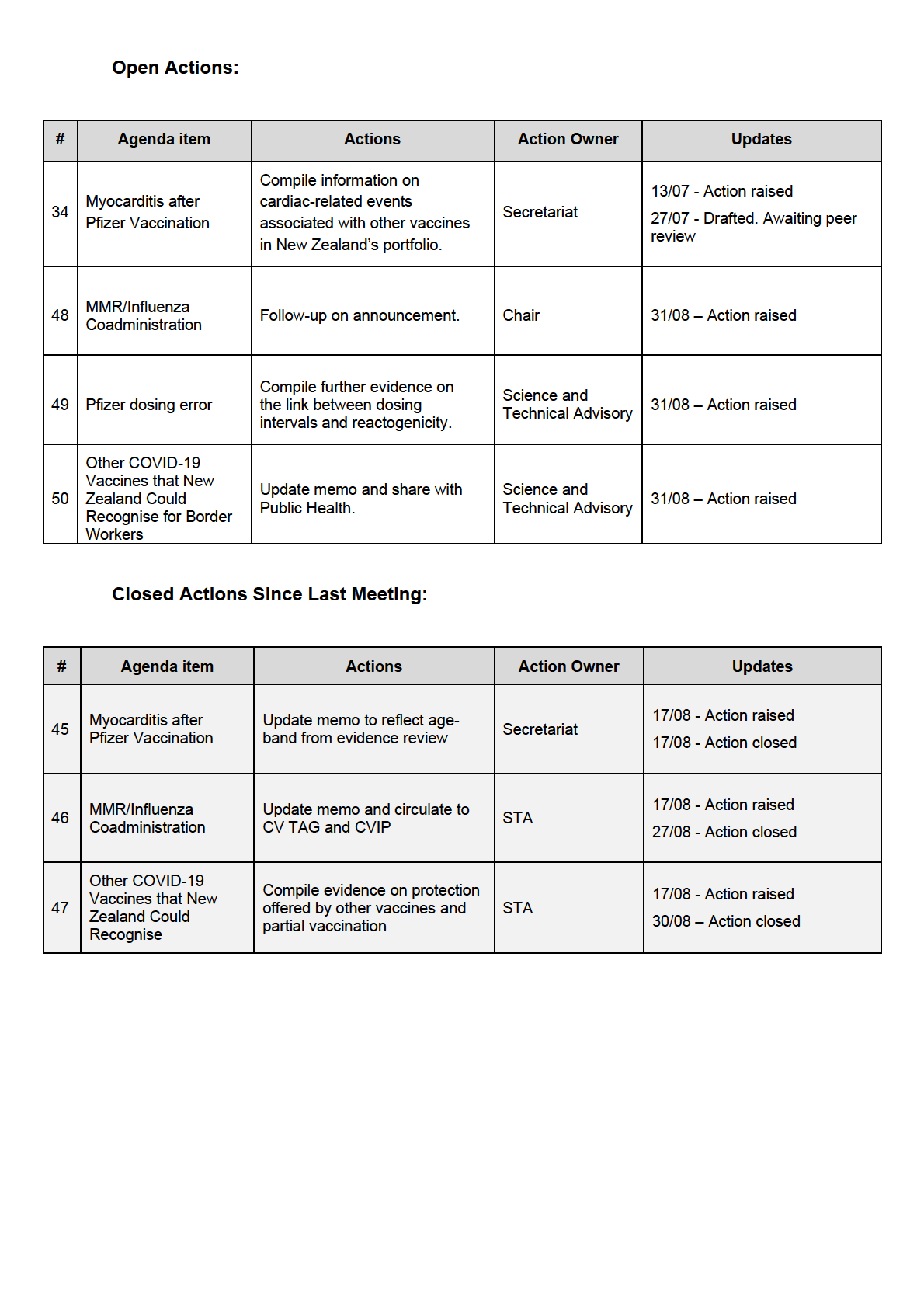
13.0
#
Agenda item
Actions
Action Owner
Updates
RELEASED
Compile information on
cardiac-related events
13/07 - Action raised
Myocarditis after
34
associated with other
Secretariat
Pfizer Vaccination
27/07 - Drafted. Awaiting
vaccines in New
peer review
Zealand’s portfolio.
 1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
 1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
 MINUTES: COVID-19 Vaccine Technical Advisory Group
Date:
MINUTES: COVID-19 Vaccine Technical Advisory Group
Date:
Tuesday 31 August 2021
Time:
11:00am to 12:00pm
Location:
Teams: 9(2)(k)
Chair:
Ian Town
Elizabeth Wilson, James Ussher, Helen Petousis-Harris, Ian Frazer, Nikki
Members:
Moreland, Nikki Turner, Peter McIntyre, Sue Crengle, Tony Walls
Andi Shirtcliffe, Brooke Hollingshead, Daniel Bernal, David Murdoch, Edwin
ACT 1982
Ministry of Health Attendees:
Reynolds, Fiona Callaghan, Juliet Rumball-Smith, Niki Stefanogiannis, Pippa
Scott, Shayma Faircloth
Guests:
John Tait, Kris Golding
Apologies:
Caroline McElnay, Sean Hanna
INFORMATION
Welcome and previous minutes
1.0
Ian Town welcomed all Members and Attendees in his capacity as Chair of the COVID-19 Vaccine
Technical Advisory Group (CV TAG), including Mr John Tait, Chair of the Vaccine ISMB.
Minutes of the last meeting (17 August 2021) were accepted.
Vaccine Rollout Update
2.0
The daily vaccine report was presented to CV TAG:
•
The rollout is proceeding at pace with high demand for vaccines. Drivers include more primary
care providers, drive-through models, and reduced waiting time post vaccination.
•
Over one million people are now fully vaccinated, and vaccines will be open to everyone from 1
September.
•
The high demand for vaccines means additional supplies may be needed from mid-September
onwards, and discussions are underway on how to source these.
•
Additional funding is being provided to support Māori and Pacific provider-led vaccination and
RELEASED UNDER THE OFFICIAL
wraparound services.
MMR/Influenza Coadministration
3.0
An update on CV TAG’s advice on the coadministration of the COVID-19 vaccine with other vaccines was
given:
•
Finalised CV TAG advice recommending that the interval between administering the COVID-19
vaccines and other vaccines be removed (with the exception of the shingles vaccine Zostavax)
has been shared with CVIP.
•
Advice will be formally announced a Steering Group.
Myocarditis after Pfizer Vaccination
4.0
The recent death of a woman with myocarditis post-vaccination was discussed with CV TAG:
• ISMB determined that vaccination was one of the causal factors.
• It was noted that this myocarditis following vaccination is extremely rare.
• The case is under review by a coroner and the case report will be published providing greater
detail.
Third Dose
5.0
This item was discussed with the agenda item below.
Pfizer Dosing Error
6.0
A draft protocol was shared with CV TAG for providing guidance for incidents where a vaccination may
1982
have been missed:
• The protocol is intended to be generic clinical guidance that can be applied to multiple situation
ACT
and will also inform guidance for the potential missed vaccination incident at Highbrook.
• It was discussed that smaller incidents should be managed under individualised clinical
management plans, and a broader approach was needed for larger groups, with an allowance for
clinical discretion.
• For large groups, in general, third doses will be offered to all of those potentially affected.
• Serology is of limited use for large groups due to high false negatives. Serology could be
INFORMATION
considered with smaller groups and if first dose was missed.
• Further evidence on the link between dosing intervals and reactogenicity was requested from the
Science and Technical Advisory team.
• The memo will be updated and shared with CVIP.
•
OFFICIAL
The group also noted generally that there is good evidence on the safety and immunogenicity
associated with administering third doses to the immunocompromised.
THE
Other COVID-19 Vaccines that New Zealand Could Recognise for Border Workers
7.0
Draft recommendations were shared with CV TAG on which vaccines could be recognised for work at the
border:
UNDER
• The group noted the need for high degrees of protection for Border Workers to reduce the risk of
onward transmission
• It was discussed that, in general, New Zealand should recognise vaccines approved by Medsafe
and Medsafe-recognised regulators: TGA, EMA, FDA, MHRA, Health Canada, and EU member
states.
•
RELEASED
One exception to the above is that border workers that have received the single-dose adenovirus
vaccine from Janssen/J&J, and no further COVID-19 vaccination, would require one dose of
Pfizer to increase their level of protection.
• Under this approach, as of 31 August, the following vaccines would be recognised for border
work: Pfizer, AstraZeneca (approved by Medsafe); Moderna, Covishield (approved by Medsafe-
recognised bodies); and Janssen/J&J plus one dose of Pfizer.
• Partial and full vaccination with vaccines not recognised by these authorities should be given a
single booster dose of the Pfizer vaccine.
 1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
 1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
 MINUTES: COVID-19 Vaccine Technical Advisory Group
Date:
MINUTES: COVID-19 Vaccine Technical Advisory Group
Date:
Tuesday 07 September 2021
Time:
11:00am to 12:00pm
Location:
Teams: 9(2)(k)
Chair:
Ian Town
David Murdoch, Elizabeth Wilson, Helen Petousis-Harris, Ian Frazer, James
1982
Members:
Ussher, Nikki Moreland, Nikki Turner, Peter McIntyre, Sue Crengle, Tony Walls
Andi Shirtcliffe, Brooke Hollingshead, Daniel Bernal, Edwin Reynolds, Fiona
ACT
Ministry of Health Attendees:
Callaghan, Shayma Faircloth
Jared Solloway, Jono Hoogerbrug, Kath Blair, Kris Golding, Maria Cotter,
Guests:
Muhammad Mulla, Sarah Jefferies
Caroline McElnay, Juliet Rumball-Smith, John Tait, Niki Stefanogiannis, Pippa
Apologies:
Scott, Sean Hanna,
INFORMATION
Welcome and previous minutes
1.0
Ian Town welcomed all Members and Attendees in his capacity as Chair of the COVID-19 Vaccine
Technical Advisory Group (CV TAG).
OFFICIAL
Minutes of the last meeting (31 August 2021) were accepted subject to the correction of attendance, with
David Murdoch listed as Member and not listed as a Ministry of Health Attendee.
THE
Influenza Programme 2022
2.0
CV TAG advice was asked to comment on aspects of 2022 Influenza Programme planning:
• There is potentially a high public health risk once borders begin to open with increased
UNDER
vulnerability.
• A public/private joint model was seen as incompatible with public health principles and therefore
universal access would be more effective, with prioritisation of vulnerable populations.
• CV TAG recommended ‘ring-fencing’ vulnerable people by vaccinating their families and
households around them for greater protection.
RELEASED
• Māori and Pacific Peoples were also needing to be prioritised based on increased vulnerability to
infection, being more likely to work in the essential workforce, and live in intergenerational
households. Whānau-based approaches could be considered to encourage uptake, as is working
well with the COVID-19 vaccine. Data on hospitalisation and mortality by ethnicity should be
included in the recommendations.
• Prioritising children and adolescents from aged 6 months to 18 years was also suggested, noting
this would also reduce the burden in older people.
• A strategic approach to the whole programme including measles, HPV and other campaigns was
called for. The National Immunisation Solution will be in place in time for the influenza programme
in 2022 and will be accessible for all providers.
Guidance for Cancer Patients
3.0
Guidance from the Cancer Control Agency on the increased vulnerability of immunocompromised patients
due to their lower vaccine response was presented to CV TAG for noting.
Third Dose for Immunocompromised
4.0
Draft recommendations of administering additional doses to the immunocompromised were presented to
CV TAG for discussion:
• CV TAG noted that the recommendations need to be a clearly defined, evidence-based, list of
conditions, including medications that may need to be listed e.g. corticosteroids.
1982
• The IMAC list of immunocompromised groups could form the basis of the list of conditions, and
recommendations for COVID-19 vaccines should be aligned with IMAC information.
ACT
• The recommendations must also outline the consent process and note that any authorised
prescriber or medical practitioner will be able to administer doses.
• This is an opportunity to reiterate that immunocompromised people are not ineligible for COVID-
19 vaccination.
• A subgroup of CV TAG will meet to revise the recommendations, and this will be brought back to
CV TAG next week.
INFORMATION
Vaccines Recognised for Border Workers
5.0
The recommendations for vaccines recognised for Border Work has been finalised and shared with the
Public Health Policy team.
Vaccines Recognised for Returnees
6.0
OFFICIAL
CV TAG advice was sought from the Public Health Policy team on the list of vaccines that could be
recognised for returnees.
THE
• The recommendations for vaccines recognised for Border Work has been finalised and shared
with the Public Health Policy team.
• In the context of New Zealand pursuing an elimination strategy with a population not yet fully
protected by vaccination, CV TAG noted that a high level of protection was still needed. Within
UNDER
this context, no vaccine currently provides enough protection to remove public health measures
or MIQ requirements completely. The list of vaccines recognised by Health Canada was noted as
an example of an approach New Zealand could follow.
• Equity issues were noted as of importance for people arriving to New Zealand, particularly with
our Pacific neighbours and RSE workers.
RELEASED
• A memo containing a list of recognised vaccines will be drafted, circulated to CV TAG for
approval, and then shared with the Public Health Policy team.
• CV TAG will continue to monitor all relevant information (including vaccine efficacy, variants,
booster and/or third doses) and will update their recommendations.
Next Steps/Decisions Pending
7.0
None.
 1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
 1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
 1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
 MINUTES: COVID-19 Vaccine Technical Advisory Group
Date:
MINUTES: COVID-19 Vaccine Technical Advisory Group
Date:
Tuesday 14 September 2021
Time:
11:00am to 12:00pm
Location:
Teams: 9(2)(k)
Chair:
Ian Town
David Murdoch, Elizabeth Wilson, Helen Petousis-Harris, Ian Frazer, James
1982
Members:
Ussher, Nikki Turner, Peter McIntyre, Sean Hanna, Tony Walls
Andi Shirtcliffe, Brooke Hollingshead, Chriselle Braganza, Daniel Bernal, Edwin
ACT
Ministry of Health Attendees:
Reynolds, Fiona Callaghan, Juliet Rumball-Smith, Pippa Scott, Shayma Faircloth
Guests:
John Tait, Maria Cotter
Caroline McElnay, Kris Golding, Niki Stefanogiannis, Nikki Moreland, Sue
Apologies:
Crengle
INFORMATION
Welcome and previous minutes
1.0
Ian Town welcomed all Members and Attendees in his capacity as Chair of the COVID-19 Vaccine
Technical Advisory Group (CV TAG).
Minutes of the last meeting (07 September 2021) were accepted.
OFFICIAL
Science Updates
2.0
THE
The Science and Technical Advisory provided an update on New Zealand’s vaccine candidates:
• There are no new data on Novavax, including on its use as a potential booster dose or in a
heterologous schedule. Medsafe continues to wait for further evidence as part of its application.
•
UNDER
Data has emerged for Pfizer that longer intervals between doses produce higher antibody titres
but lower T Cell responses. There is also some evidence that immunity may wane markedly in
the elderly, and some further evidence on vaccine efficacy against Delta.
• Pfizer is now fully approved in Switzerland, the US, Brazil and Japan.
• One article reported a higher risk of myocarditis for 12-15-year-olds in data from the US,
however CV TAG noted there were significant issues with the data, and they await further
RELEASED
information.
Research in Children
3.0
This item was covered under agenda item 2.0 Science Updates.
Vaccine Rollout
4.0
The Chair provided an update on the vaccine rollout to CV TAG:
• The outbreak continues to dominate much of the work at the Ministry, however, the vaccine
rollout continues at pace. The programme has adopted CV TAG’s advice on using vaccines as a
control measure in an outbreak through strong advice to Aucklanders to get vaccinated and
greater efforts to roll the vaccine out in Auckland focussing on Pacific and Maori communities.
•
Further supply has now been secured from Spain and Denmark.
•
Data on vaccination rates for Māori and Pacific Peoples were shared showing some
improvement in uptake.
Third Dose for Immunocompromised
5.0
•
A subgroup of CV TAG met to revise the recommendations on administering additional doses to
the immunocompromised.
•
The UK’s Joint Committee on Vaccination and Immunisation (JCVI) criteria for
immunocompromise was noted as a clear and prescriptive set of criteria that New Zealand could
follow capturing individuals with severe immunocompromise
•
Other important measures for the protection of the immunocompromised include: ‘ring-fencing’
vulnerable people by vaccinating household members; continuing other public health measures
(such as masking).
ACT 1982
•
The additional dose is a ‘top-up’ or third primary dose, as opposed to a booster dose.
•
Serology is not considered a useful tool, as a correlate of protection has not been established,
among other reasons.
•
Discussion with Medsafe will be required in order to implement the additional dose.
•
In general, the additional dose is to be given 8 weeks or more after the second dose.
•
The JCVI recommendations will be further checked to ensure they align with the IMAC
INFORMATION
handbook for special groups, finalised, and shared with CVIP. It could be added to the
Immunisation Handbook.
•
The extension dose protocol will also be updated to refer to this definition of
immunocompromise, rather than the CDC list that was used prior.
Vaccines Recognised for Arrivals
6.0
•
Advice was sought on which vaccines would be required for travellers during the phased easing
of border restrictions and whether the standard for Border Workers could apply, or whether the
broader WHO list could be recognised (with Sinopharm and Sinovac).
•
In general, the broader WHO list was considered to provide an acceptable level of protection for
people arriving to the country. All these vaccines offer some protection against severe disease.
However, people fully or partially vaccinated with Sinovac and Sinopharm may need an
additional dose of the Pfizer vaccine to gain sufficient protection.
•
Other considerations include: the requirements for children and adolescents (aged 12-15); the
requirements for an additional dose for individuals already in the country who have received
Sinopharm or Sinovac
RELEASED UNDER THE OFFICIAL
•
Vaccine recognition policies for Border Workers should also be considered for healthcare
workers as they also work in high-exposure settings.
•
A further and separate discussion is needed on Janssen as any decisions on additional dose
requirements for arrivals may impact on the decision to use more broadly.
•
CV TAG will continue to monitor emerging evidence. The recommendations on vaccines to be
recognised will be brought back to CV TAG prior to the pathways being finalised.
•
Preliminary advice that all inbound travellers going into MIQ from 1 November should have been
vaccinated (with any vaccine) was noted and supported.
 1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
 1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
 MINUTES: COVID-19 Vaccine Technical Advisory Group
Date:
MINUTES: COVID-19 Vaccine Technical Advisory Group
Date:
Tuesday 21 September 2021
Time:
11:00am to 12:00pm
Location:
Teams: 9(2)(k)
Chair:
Ian Town
David Murdoch, Elizabeth Wilson, Helen Petousis-Harris, Ian Frazer, James
1982
Members:
Ussher, Nikki Moreland, Nikki Turner, Peter McIntyre, Sue Crengle, Tony Walls
ACT
Brooke Hollingshead, Chriselle Braganza, Daniel Bernal, Edwin Reynolds, Fiona
Ministry of Health Attendees:
Callaghan, Juliet Rumball-Smith, Niki Stefanogiannis, Pippa Scott, Shayma
Faircloth
Guests:
Kris Golding, Maria Cotter
Apologies:
Andi Shirtcliffe, Caroline McElnay, John Tait, Sean Hanna
INFORMATION
1.0
Welcome and previous minutes
Ian Town welcomed all Members and Attendees in his capacity as Chair of the COVID-19 Vaccine
Technical Advisory Group (CV TAG).
OFFICIAL
Minutes of the last meeting (14 September 2021) were accepted.
2.0
Vaccine Rollout
THE
The Chair provided an update on the vaccine rollout:
• Increasing access to vaccination in suburbs affected by the current outbreak is a focus currently.
• A range of initiatives are underway (e.g., mobile vaccine buses), with discussions about
incentives and ways to reduce barriers.
UNDER
• Discussions are occurring with the Ministry of Education about administering vaccines to 12–15-
year-olds and their families in education settings
• Progress with vaccination is increasing steadily with the number of first doses administered
expected to reach 80% in the next few days.
RELEASED
3.0
Third Dose for Immunocompromised
The draft memo with recommendations for severely immunocompromised people to receive an additional
dose of the Pfizer vaccine was shared with CV TAG.
• CV TAG’s recommendations align with advice given in the IMAC handbook for the severely
immunocompromised. STA will keep a watching brief on the other conditions included in the
IMAC handbook associated with non-severe immunocompromise, namely asplenics, diabetes
and dialysis. These will be updated as further evidence emerges.
• Medsafe and the Ministry of Health’s legal team have reviewed the definition of who can
administer the additional dose.
• The Cancer Control Agency have been consulted and agreed that the CV TAG advice aligns with
their advice regarding severe immunocompromise.
• The dose wil be framed as an ‘additional dose’ for clarity.
• The advice will be signed out and shared with CVIP and IMAC.
4.0
Vaccines Recognised for Arrivals
• A draft memo was presented to CV TAG with recommendations that from 1 November, everyone
entering 14 days MIQ in New Zealand will need to be vaccinated. The memo specifies that:
o Arrivals should have had a full course with one of the 22 vaccines approved by
regulatory authorities or governments around the world, at least 14 days prior to arrival.
o Those vaccinated with a non-WHO vaccine will require an additional dose of the Pfizer
vaccine on leaving MIQ.
o An exemption process will be available for countries without access to vaccines for 12-
1982
15-year-olds, who will be offered Pfizer vaccination.
o Vaccine status will be self-reported with any form of proof accepted by the airline at
ACT
check-in, and on arrival at customs.
o The purpose of introducing vaccine requirements for MIQ is not to stop transmission into
the community, but rather about allowing equitable entry, and protection to the same
extent as others in New Zealand.
• Between 24 August and 17 September 2021, of the 2,438 MIQ guests during this period, 2,218
(91%) were fully vaccinated, and only 14 people (0.6%) were unvaccinated, and therefore it is
expected to affect a small proportion of people.
• Some concern was raised about the efficacy of Sinopharm and Sinovac.
INFORMATION
• Data was also requested on the positivity rate of tests at Day 3 and 10 in MIQ, and Day 6 when
available. Shortened MIQs for vaccinated travellers will be discussed at a later date.
• Additional doses should be administered as soon as possible once people arrive to New
Zealand, with the advantage of time in MIQ being utilised. At the latest, they could be
administered on leaving MIQ. Additional doses after leaving MIQ would result in inequities in
OFFICIAL
uptake and access. It was noted that there were workload and operational concerns with
administering doses while in MIQ.
• The requirement of having to have been vaccinated at least 14 days prior to arriving to MIQ was
THE
considered to be unnecessarily restrictive.
• The issue of whether healthcare workers vaccinated with Janssen should receive an extra dose
of Pfizer was raised, due to the enhanced need for protection of a high-risk occupation. This will
feed into broader work on vaccines, including vaccines to recognise for seasonal workers and
UNDER
those for new arrivals as part of the traveller-risk pathways. The evidence in this area is evolving
and therefore STA and CV TAG will continue to monitor new information as it emerges and make
updates as required.
5.0
Third Booster Doses
RELEASED
• The recommendations made by the UK’s Joint Committee on Vaccination and Immunisation to
administer booster doses to all aged over 50 were brought to CV TAG for discussion.
• It was flagged that evidence is accumulating on waning in the elderly. Those aged over 65 and/or
vulnerable subgroups are likely to need a booster dose. However, it is still unclear when this
should occur and in which subpopulations, and further evidence is required.
• The STA team will begin a work programme to start building the evidence base for potential
booster doses in the elderly, and this will be brought back to CV TAG.
6.0
Decision to use for 12–15-year-olds
• Considering the UK’s decision to not vaccinate this age group, it was queried whether this
decision should be revisited, and/or for only single doses to be administered.
• Aotearoa New Zealand’s population is immunologically naïve and therefore it is still important
that this population is vaccinated with two doses.
• However, greater emphasis is needed on the benefits provided by longer dosing intervals, with
CV TAG expressing concern that intervals of 3 weeks were becoming more common in
Auckland’s outbreak.
• The opportunity for CV TAG position statements to be shared publicly was noted as something
that could be explored in order to reinforce the current recommendation of 6 weeks.
• The new Pfizer results released showing a robust immune response in 5–11-year-olds given a 2
lower doses of the Pfizer vaccine were discussed. CV TAG will continue to follow the evidence as
it emerges and raise any questions when meeting with Pfizer this week.
• No change to the current guidance.
1982
7.0
Next Steps/Decisions Pending
ACT
None.
8.0
Any Other Business
Concern was raised with Dr Shane Reti incorrectly commenting on RNZ (21 September) that an interval
of 1 week was being considered, with the vaccine not being approved by Medsafe for this interval.
INFORMATION
Engagement with his office is required.
9.0
Agenda items for next meeting
Vaccines recognised for MIQ entry
OFFICIAL
Vaccines recognised for Recognised Seasonal Employer (RSE) workers
THE
10.0
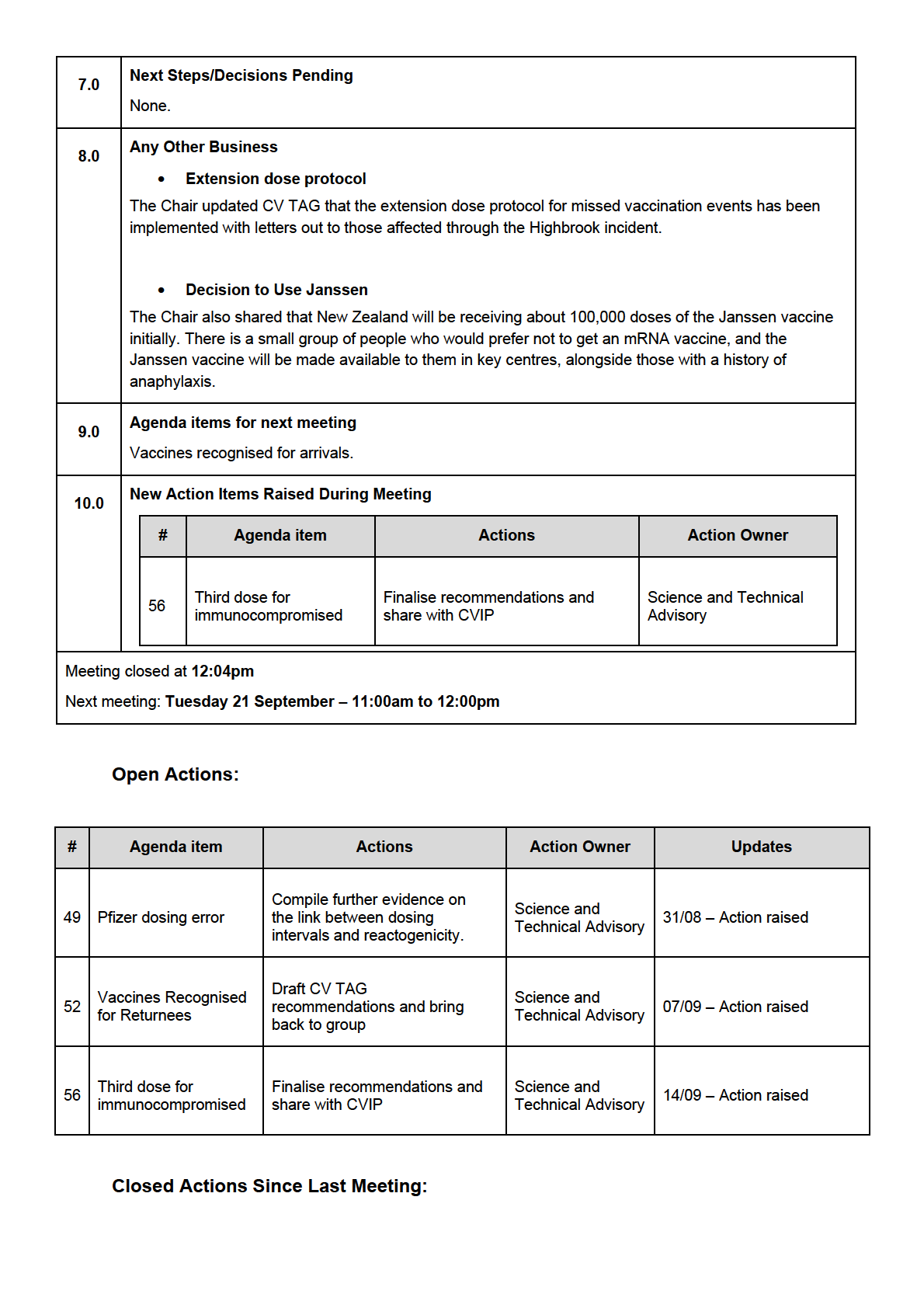
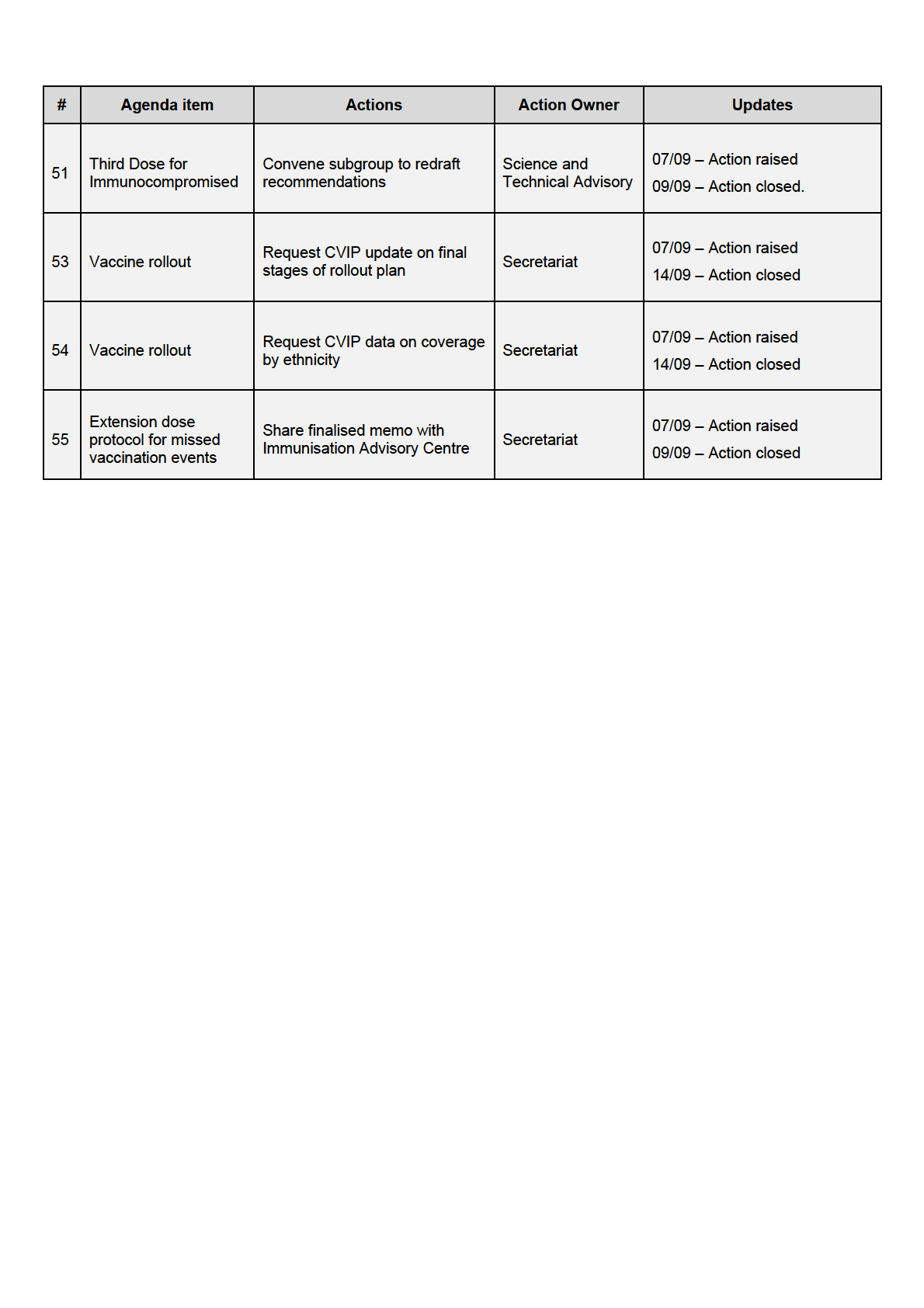
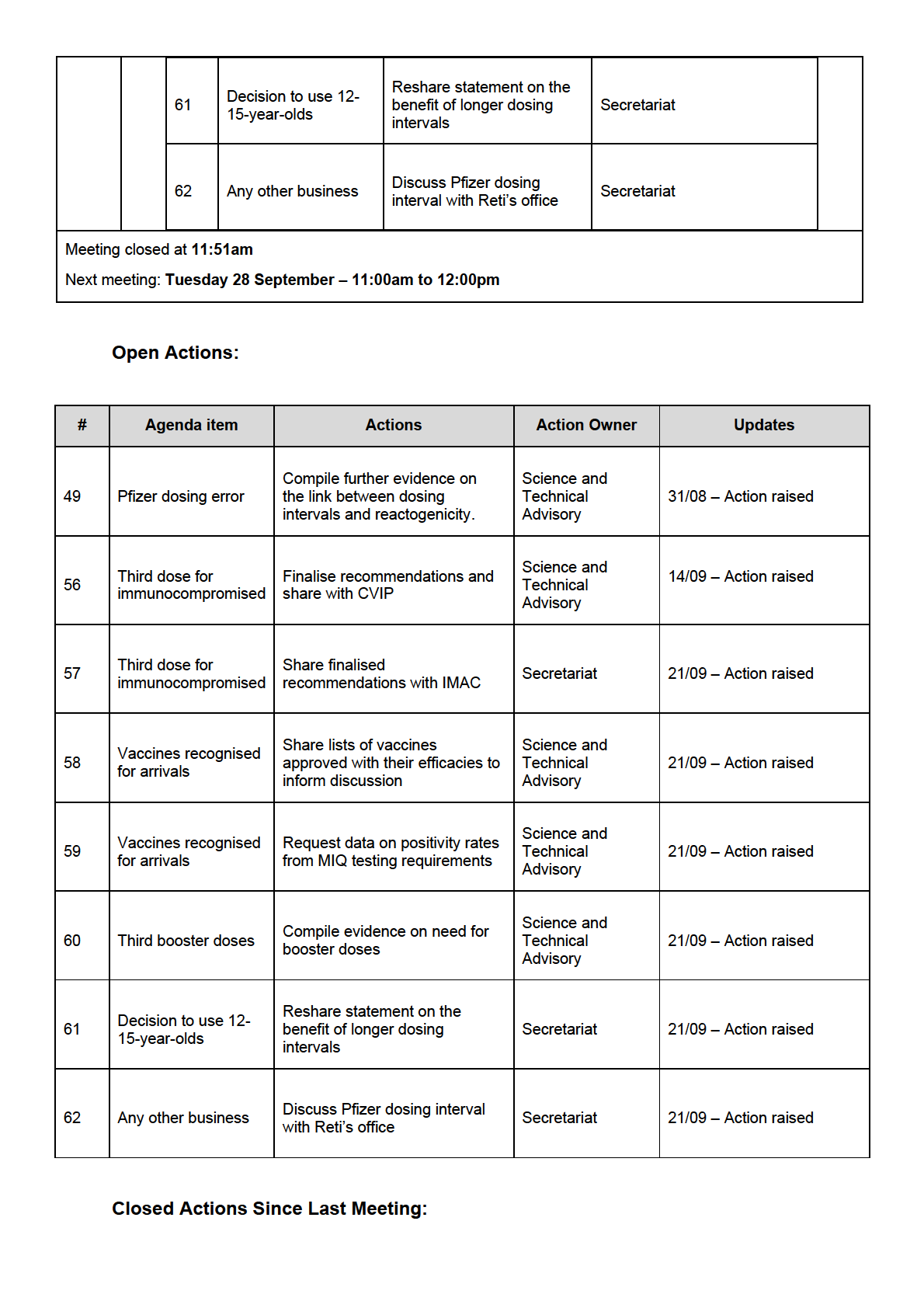
New Action Items Raised During Meeting
UNDER Share finalised
Third dose for
57
recommendations with
Secretariat
immunocompromised IMAC
Share lists of vaccines
Vaccines recognised
approved with their
Science and Technical
58
for arrivals
efficacies to inform
Advisory
RELEASED
discussion
Request data on positivity
Vaccines recognised
Science and Technical
59
rates from MIQ testing
for arrivals
Advisory
requirements
Compile evidence on need
Science and Technical
60
Third booster doses
for booster doses
Advisory
 1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
 1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
 MINUTES: COVID-19 Vaccine Technical Advisory Group
Date:
MINUTES: COVID-19 Vaccine Technical Advisory Group
Date:
Tuesday 05 October 2021
Time:
11:00am to 12:00pm
Location:
Teams: 9(2)(k)
Chair:
Ian Town
David Murdoch, Elizabeth Wilson, Ian Frazer, James Ussher, Nikki Moreland,
1982
Members:
Peter McIntyre, Sean Hanna, Sue Crengle, Tony Walls
Andi Shirtcliffe, Brooke Hollingshead, Chriselle Braganza, Daniel Bernal,
ACT
Ministry of Health Attendees:
Edwin Reynolds, Erin Smith, Fiona Callaghan, Juliet Rumball-Smith, Pippa
Scott
Guests:
Kris Golding, Mariana Traslosheros Reyes
Caroline McElnay, Helen Petousis-Harris, John Tait, Niki Stefanogiannis,
Apologies:
Nikki Turner
INFORMATION
1.0
Welcome and previous minutes
Ian Town welcomed all Members and Attendees in his capacity as Chair of the COVID-19 Vaccine
Technical Advisory Group (CV TAG).
OFFICIAL
Minutes of the last meeting (21 September 2021) were accepted.
2.0
Vaccine Rollout
THE
The Chair provided an update on the vaccine rollout:
• The vaccine rollout continues to gather momentum, and further work is underway to engage with
at-risk communities through local providers and a focus on providing encouragement to those
UNDER
who are hesitant about getting the vaccine.
• It has been agreed that the default booking rules change back to a three-week interval, due to
the changing context of the Delta outbreak and the increased potential for circulating virus, there
is an increased need to get second doses administered
• The shift of resources to administering second doses was seen as anti-equity as it may divert
RELEASED
focus from outreach to Māori and Pasifika who have not yet had first doses, however it was noted
there is no shortage of vaccines or appointments to do both.
• There was some discussion on whether a longer interval should be kept for adolescents and
young people <30 due to wanting more data on the connection between intervals and side
effects.
• A shift back to three-week intervals would likely see an increase in people receiving their second
dose before the minimum of 21 days, and therefore continued communication on the minimum
interval between doses was needed.
3.0
Vaccines recognised for MIQ entry and RSE workers
• Recommendations on the vaccination requirements for entering MIQ have been sent to CVIP. A
person can enter MIQ if they have been fully vaccinated with the COVID-19 vaccines approved
by at least one government or authority around the world. Those who have been vaccinated with
a vaccine that is not approved by Medsafe or a Medsafe-approved authority will be offered an
additional dose of the Pfizer vaccine.
• Recommendations on the vaccine requirements for RSE workers arriving to New Zealand have
been shared with Global Health and the Realm countries. While RSE workers were encouraged
to be fully vaccinated before arriving, some will arrive having only had one dose. RSE workers
who have had a full course of AstraZeneca are considered fully vaccinated. Those who have only
had one dose of AZ, or who have been vaccinated with Sinopharm (one or two doses) will be
offered an additional dose of Pfizer.
4.0
Supporting evidence for Health Care Worker vaccination order
1982
Evidence in support of the mandatory vaccination of healthcare workers was reviewed by CV TAG:
ACT
• The evidence was largely focussed on experience the Delta VOC and the benefits of the Pfizer,
vaccine, however other vaccines were also included in case healthcare workers may have been
vaccinated in other countries with other vaccines.
• A high level of individual protection against infection and disease is offered by the Pfizer vaccine.
This was seen as of importance to protect healthcare workers but also to ensure workforce
capacity remains steady.
• Preliminary evidence of the impact of vaccination on transmission is promising although
INFORMATION
protection against transmission may wane. Further evidence on this will be reviewed, with a
particular focus on the impact of furloughing healthcare workers due to their being contacts.
5.0
VAANZ vaccine candidate development update and Research Project
OFFICIAL
An update was provided on the VAANZ vaccine candidates and research:
• VAANZ now have two second generation COVID-19 vaccine candidates in the process of
THE
advancing to manufacturing: An adjuvant sub-unit protein booster vaccine targeted to the Delta
variant, and a pan-coronavirus vaccine in development with Trans-Tasman partners, as part of
an mRNA platform to protect broadly across coronaviruses
•
UNDER
Phase 1 clinical trials for each of these candidates are expected to be running by early 2023.
• Research is underway to assess immunogenicity of the COVID-19 vaccine in recipients aged
over 16, and to assess differences in the immune response by ethnicity, age, and presence of
comorbidities. The study is fully-enrolled (302 recruited) including 29% Māori and 30% Pacific
Peoples. Data is expected in December 2021.
RELEASED
6.0
BMI needle length study update
An update was also provided by the Ministry’s Post-Events team on the BMI needle length study:
• Recruitment is underway with about 100 participants currently recruited from the Mt Wellington
vaccination centre.
• However, the current lockdown restrictions in Auckland have provided challenges and further
funding has been requested from the Ministry of Health. A budget reforecasting is underway, and
the project will have a longer run time.
 1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
 1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
 MINUTES: COVID-19 Vaccine Technical Advisory Group
Date:
MINUTES: COVID-19 Vaccine Technical Advisory Group
Date:
Tuesday 19 October 2021
Time:
11:00am to 12:00pm
Location:
Teams: 9(2)(k)
Chair:
Ian Town
David Murdoch, Elizabeth Wilson, Helen Petousis-Harris, James Ussher, Nikki
1982
Members:
Moreland, Peter McIntyre, Sean Hanna,
ACT
Andi Shirtcliffe, Brooke Hollingshead, Chriselle Braganza, Edwin Reynolds, Erin
Ministry of Health Attendees:
Smith, Fiona Callaghan, Juliet Rumball-Smith, Pippa Scott
Guests:
Chris James, John Tait, Kris Golding, Susan Kenyon, Ralph Stewart
Caroline McElnay, Daniel Bernal, Ian Frazer, Niki Stefanogiannis, Nikki Turner,
Apologies:
Sue Crengle, Tony Walls,
INFORMATION
1.0
Welcome and previous minutes
Ian Town welcomed all Members and Attendees in his capacity as Chair of the COVID-19 Vaccine
Technical Advisory Group (CV TAG).
Minutes of the last meeting (05 October 2021) were accepted.
OFFICIAL
2.0
Vaccine Rollout and Outbreak THE
The Chair provided an update on the vaccine rollout:
• ‘Super Saturday’ on October 16 provided a major boost to the vaccination rollout with
approximately 130,000 doses administered, and many doses were among Māori and younger
adults. All data broken down by DHB is publicly available on the Ministry of Health website.
UNDER
3.0
Supporting Evidence for Healthcare Worker Vaccination Order
• The evidence brief that CV TAG provided input into to support the mandatory vaccination of
healthcare workers is being finalised. A brief evidence summary was included with the Cabinet
paper, focussing on the effect of vaccination on transmission.
RELEASED
• The specifics of any exemption policy were discussed. A small group of people may be medically
exempt from the Pfizer vaccine, however, having an alternative vaccine available may also be of
interest to other groups eg, healthcare workers.
• The finalised evidence brief from CV TAG will be signed out as a memo and shared with CVIP.
4.0
Decision to Use AstraZeneca
• The AstraZeneca vaccine may be considered for people who are unable to take the Pfizer
vaccine due to contraindications, or due to issues with their first dose, as well as those hesitant
about getting an mRNA vaccine.
• The vaccine was considered suitable for anyone eligible and indicated as per the Medsafe data
sheet, however it was noted that the data sheet had no age restrictions in its indication, nor
prescribed dosing intervals.
• AstraZeneca has been used with a range of dosing intervals (e.g., 4-12 weeks), though some
countries have reduced this to four weeks in an outbreak.
• The risk of thrombosis and thrombosis with thrombocytopaenia was noted as a concern, with
incident rates higher among younger adults. AusVaxSafety provide comparative data by age for
AstraZeneca and Pfizer and would be a useful resource. It was also noted that the vaccine has
not been trialled or used among pregnant people.
• Possible distribution channels for the different groups were queried. Distribution will likely be
limited to certain centres to reduce the risk of error and due to larger volumes of the vaccine
being needed to avoid waste. Those who had had an adverse event after their first dose could be
referred through primarycare. People with a preference for a non-mRNA vaccine could be
1982
directed to certain vaccine centres with supplies or receive a booking code.
• The STA team will draft recommendations for CV TAG to consider this week based on the
ACT
Medsafe data sheet and data internationally.
• The Ministry of Health’s Policy team may seek advice on Janssen, Novavax or AstraZeneca at a
later date.
5.0
Myocarditis Update
• An update was provided from STA on the risk of myocarditis according to international evidence.
Data presented at the latest US ACIP meeting on 30 August 2021 and data from Israel indicate
that myocarditis reporting rates following mRNA COVID-19 vaccination continue to be rare
INFORMATION
overall, but highest risk tends to occur after the second dose, particularly in younger males.
• Medsafe also shared the latest data on cases. The safety profile differs to the US in that New
Zealand is seeing more cases after dose 1 than dose 2, however this could reflect the vaccine
rollout with more young people being vaccinated later. Onset tends to be reported in the first five
OFFICIAL
days for both dose. Data on dosing intervals has not been analysed, however it has been noted
that cases have still occurred at an interval of 6-8 weeks. Overall, the rate is approximately 7 per
million doses after dose 1, and 10 per million doses after dose 2. Peopled aged 30-39 are the
THE
most affected age group in New Zealand overall, and after dose 1, and people aged 20-29 are
most affected after dose 2. Long-term follow-up data is expected by end of November.
• ISMB shared that levels of reporting seem to correlate with the numbers of reports being
received, looking at the number of hospitalisations in vaccinated individuals. Every case reported
UNDER
to CARM is reviewed by a medical assessor, and when there is insufficient data, further
information is requested. If there is a risk of death, biopsies and post-mortems of myocardiums
are requested. No long-term outcome data is currently available.
• Information on symptoms to watch out for have been provided to all vaccinators, however it is
possible that some centres are still using older booklets from before the advice was given.
RELEASED
• Milder cases may benefit from further clinical investigation, and greater standardisation in
management of care may be needed with ECGs and provision of troponins. Accessiblity of the
guidance for general practice and primary care will be reviewed.
• As previously noted, people who have myocarditis after their first dose should not be offered a
second dose of an mRNA vaccine, and an alternative vaccine or no further doses should be
considered for those people.
• No further evidence had emerged that decreasing the dose interval had impacted myocarditis.
• A clinical research project is one option to consider looking at myocarditis in greater detail.
 1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
 1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
 1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
 MINUTES: COVID-19 Vaccine Technical Advisory Group
Date:
MINUTES: COVID-19 Vaccine Technical Advisory Group
Date:
Tuesday 02 November 2021
Time:
11:00am to 12:00pm
Location:
Teams: 9(2)(k)
Chair:
Ian Town
Elizabeth Wilson, Helen Petousis-Harris, Ian Frazer, James Ussher, Nikki
1982
Members:
Moreland, Nikki Turner, Peter McIntyre, Sue Crengle, Tony Walls
ACT
Brooke Hollingshead, Chriselle Braganza, Daniel Bernal, Edwin Reynolds, Erin
Ministry of Health Attendees:
Smith, Fiona Callaghan, Juliet Rumball-Smith, Pippa Scott
Guests:
John Tait, Kris Golding, Thomas Teunissen, Liam McConnell
David Murdoch, Sean Hanna, Andi Shirtcliffe, Caroline McElnay, Niki
Apologies:
Stefanogiannis
INFORMATION
1.0
Welcome and previous minutes
Ian Town welcomed all Members and Attendees in his capacity as Chair of the COVID-19 Vaccine
Technical Advisory Group (CV TAG).
Minutes of the last meeting (19 October 2021) were accepted.
OFFICIAL
2.0
Vaccine Rollout and Outbreak THE
• Vaccine uptake continues to increase. Vaccination rollout data and case details are available on
the Ministry of Health website.
3.0
Decision to Use AstraZeneca
UNDER
• The finalised recommendations have been shared with the Director-General and CVIP, and the
team is now working on acquiring doses of the AstraZeneca vaccine. As recommended by CV
TAG, this vaccine will be targeted to those who are contraindicated to the Pfizer vaccine, or
hesitant about receiving an mRNA vaccine.
• Details of implementation will be brought back to CV TAG to outline delivery dates and how it will
RELEASED
be operationalised.
• Doses of the Janssen vaccine are still expected in early 2022.
4.0
Medical exemptions
Draft recommendations on the clinical criteria for temporary medical exemptions to the vaccine were
discussed.
• The recommendations were drafted based on ATAGI advice, and are intended to be temporary
exemptions lasting for a maximum of six months.
• The recommendations limit medical exemptions to a narrow group of people including: people who
have had anaphylaxis to the first dose, inflammatory cardiac illness, PCR-confirmed infection, a
serious adverse event to prior dose, or for people who are unable to tolerate vaccination (e.g.
people with severe neurodevelopment conditions).
• Once alternative vaccine(s) are available, there will be changes to the exemptions, and it will be
important to ensure that alternative vaccines are suitable e.g., the AstraZeneca ‘s TTS risk in
younger age groups should be considered.
• A temporary exemption should be included for people who experience myocarditis after the first
dose.
• A temporary exemption will be offered for people who are in clinical trials, e.g., the Valneva clinical
trial. Reasons for this include not placing an undue burden on clinical trial participants and being
unable to retrospectively impose conditions on trial participants that they have not agreed to.
• Discussion occurred on who would have the ability to grant medical exemptions, and further
1982
guidance will be sought from IMAC and the Ministry’s Clinical Quality and Safety team.
• The draft memo will be revised and finalised.
ACT
5.0
Booster doses
Draft recommendations on the clinical criteria for booster doses were discussed.
• These were based on the JCVI and ATAGI advice and New Zealand’s original prioritisation
framework.
• CV TAG requested that the criteria be simplified, and the prioritisation framework not be used, due
to New Zealand being in a different context with circulating virus, ample vaccine supply and
INFORMATION
infrastructure to deliver booster doses.
• Boosters for everyone over 30 were discussed with access to a booster dose at least 6 months
after their primary course of vaccination, however there is insufficient data on the risk and safety
for younger people at this stage.
OFFICIAL
• Prioritisation for people at high risk of severe disease (e.g., Māori), and high risk of exposure (e.g.,
healthcare workers), followed by their whānau was discussed.
THE
• Age-criteria for prioritisations raise equity concerns particularly for Māori due to the increased risk
of severe disease and hospitalisation, i.e., a lower age band for Māori should be considered to
provide equivalent protection.
• Concern was expressed that this would divert efforts and attention away from primary vaccination
UNDER
efforts, and therefore first and second doses should be prioritised over booster doses, and an
overarching statement will be added to the recommendations to this effect.
• The memo will be updated with the feedback from CV TAG and shared with CVIP once Medsafe
approval occurs.
6.0
‘Fully-vaccinated’ definition
RELEASED
• Draft recommendations on the criteria for ‘fully-vaccinated’ within the New Zealand border were
shared, with this defined as being 7 days after a complete course of a COVID vaccine.
• This would be used for vaccine certificates and in areas where vaccines are mandated within New
Zealand’s borders, and is not related to work on which vaccines would be recognised at New
Zealand’s border.
• Which vaccines will be included under these guidelines (e.g., WHO recognised vaccines vs.
vaccines recognised by a Medsafe Recognised Authority) was discussed, and which vaccines
may benefit from an additional dose.
• Heterologous schedules were seen as generally acceptable.
• There was discussion about the risks of mandating vaccinations for people at elevated risk of
adverse events e.g., younger people aged 12-17 and the increased risk of myocarditis after the
second dose, and a single dose may be sufficient
• There was also some discussion about whether younger people with a documented infection may
only need one dose.
• A finalised version of the memo will be distributed.
7.0
Immunocompromised populations and ATAGI’s update guidance
• CV TAG issued guidelines on which immunocompromised populations should be considered for a
third primary dose in September. Since then, ATAGI have updated their guidance to include some
broader groupings, and the Ministry received some feedback from rheumatology and haematology
groups.
1982
• The timing for the third primary dose will also be updated to be from 4 weeks, rather than 8 weeks,
as some flexibility is needed in relation to the timing of treatment.
ACT
• Guidance will be updated to reflect this feedback.
8.1
Research Studies: VAANZ further funding request
A proposal to extended funding for the Ka Mātau, Ka Ora Study was considered by CV TAG.
• The Ka Mātau, Ka Ora Study is assessing immunogenicity of the Pfizer vaccine in New Zealand
recipients >=16 years old and comparing immune responses by age, ethnicity and presence of co-
morbidities.
INFORMATION
• The research was seen to be of great importance to understanding differences in immune
responses for the Ministry of Health, with funding being drawn from the Ministry’s Post-Event
research funding pool.
• The extension of funding was supported.
OFFICIAL
8.2
Research Studies: Myocarditis research
THE
A request to support research myocarditis following COVID-19 vaccination was also considered.
• An ongoing long-term follow-up study was discussed regarding cases with a clinical diagnosis of
myocarditis and/or pericarditis following vaccination, as reported to CARM.
•
UNDER
CV TAG members were requested to volunteer to form a subgroup to develop plans and present a
proposal for additional research questions to the Post-Event team.
8.3
Research extension: Establishing a foundation for monitoring the safety of COVID-19 vaccines
using primary care data
A request to endorse an extension of a research project from the University of Auckland (UoA) was
RELEASED
received.
• The extension will allow the project to establish background rates of adverse events of special
interest (AESI) of COVID-19 vaccines from hospital discharge data and enable a foundation for
monitoring the safety of COVID-19 vaccines using primary care data.
• CV TAG noted that having baseline rates would be valuable to determine the safety profile of
vaccines and endorsed the proposal.
9.0
Medsafe provisional approval of the Pfizer vaccine extended
 1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
 1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
 1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED
1982
ACT
INFORMATION
OFFICIAL
THE
UNDER
RELEASED





























