
Document 1
MINUTES: COVID-19 Vaccine Technical Advisory Group
Date:
Tuesday 09 November 2021
Time:
11:00am to 12:00pm
Location:
Teams: S9(2)(k)
Chair:
Ian Town
David Murdoch, Elizabeth Wilson, Helen Petousis-Harris, Nikki Moreland, Nikki
Members:
Turner, Peter McIntyre, Sean Hanna, Sue Crengle,
Brooke Hollingshead, Caroline McElnay, Chriselle Braganza, Daniel Bernal,
ACT 1982
Ministry of Health Attendees:
Edwin Reynolds, Fiona Callaghan, Juliet Rumball-Smith, Niki Stefanogiannis,
Pippa Scott, Imogen Roth
Guests:
Kris Golding
Apologies:
Ian Frazer, James Ussher, Tony Wall, Andi Shirtcliffe, Erin Smith, John Tait
INFORMATION
1.0
Welcome and previous minutes
Ian Town welcomed all Members and Attendees in his capacity as Chair of the COVID-19 Vaccine
Technical Advisory Group (CV TAG).
Minutes of the last meeting (02 November 2021) were accepted.
2.0
Science Updates
The Science and Technical Advisory team provided an update on the Novavax
•
Novavax has submitted an application for approval to Medsafe.
•
There is still limited data other than from the clinical trials which were for people 18 years and
over. The Serum Institute of India have manufactured some Novavax vaccines, and these have
been granted emergency use authorisation in Indonesia.
•
Currently there is no data on how Novavax performs against Delta.
•
Manufacturing issues have slowed licencing in the US, however there are limited details on this
publicly available.
RELEASED UNDER THE OFFICIAL
3.0
Research in Children
The Science and Technical Advisory team provided an update on vaccine candidates for children:
•
There is new clinical trial data on the safety and efficacy of the Pfizer vaccine in 5-11-year-olds. A
favourable safety profile is evident with most reactions being mild, self-limiting, and similar to
adults. The US CDC has stated that clinical trial vaccine efficacy against symptomatic lab-
confirmed COVID-19 was 90.9%.
4.0
Booster Doses
Draft recommendations were discussed:
Document 1
• Booster doses were discussed, at 6 months or more after a primary course of vaccination for
everyone aged 18 and over, with priority groups identified.
• A need for caution among 18–30-year-olds was noted due to a potentially different benefit-risk
equation.
• Concern was expressed about mandating booster doses for employment reasons for people aged
18-30-year-olds.
• Pregnant people will only be excluded if they received their full primary course in early pregnancy.
• Implementation was discussed. Details of the rollout need to be worked through however it is likely
the booster vaccination programme will be woven into schedules and recalls in 2022.
• The booster programme should not distract from the primary vaccination programme and this has
been communicated. There is no shortage of vaccines or bookings for primary vaccination.
• An updated memo will be shared with CV TAG and will be finalised overnight
5.0
Provisional approval for Comirnaty vaccine booster dose
ACT 1982
This item was noted and covered under item 4.0.
6.0
Vaccination in 5–11-year-olds
• In general, a cautious approach to wait for more data was agreed, and this has been
communicated to the Director-General and Prime Minister ATAGI is also taking this approach.
• Some vulnerable 5-11-year-old groups may need protection. Individual risk factors such as
comorbidities and pre-existing conditions were discussed, as well as the importance of broader
INFORMATION
social determinants of health, crowded or intergenerational households, and protection for Māori
and Pacific Peoples.
• The indirect impacts of exclusions from school or recreation were also noted as being significant,
and children’s role in transmission
• The STA team will collate information on the risks and benefits, and this will be brought back to
CV TAG for discussion.
7.0
Immunocompromised Populations and Updated Advice
• Advice on immunocompromised populations eligible for a third primary dose has been updated.
Updates are be ng made to incorporate advice from: rheumatologists, the Gastro Society and
Canterbury MI group; ATAGI’s updated guidelines which are more inclusive, for example by
including dialysis patients.
• The recommendations serve as guidance and are not to be considered as strict inclusion criteria.
Therefore, some key scenarios are given as examples.
• Clinical judgement should be applied by the prescriber to determine whether someone has
sufficient immunocompromise to need a third primary dose.
RELEASED UNDER THE OFFICIAL
• Medsafe are preparing to address third primary doses.
• Updated guidance will be shared with CVIP.
8.0
Medical Exemptions Memo
• CV TAG advice on who is medically exempt from mandatory vaccination has been
operationalised, with the eligibility criteria now available on the Ministry of Health website and a
centralised application process is being established.
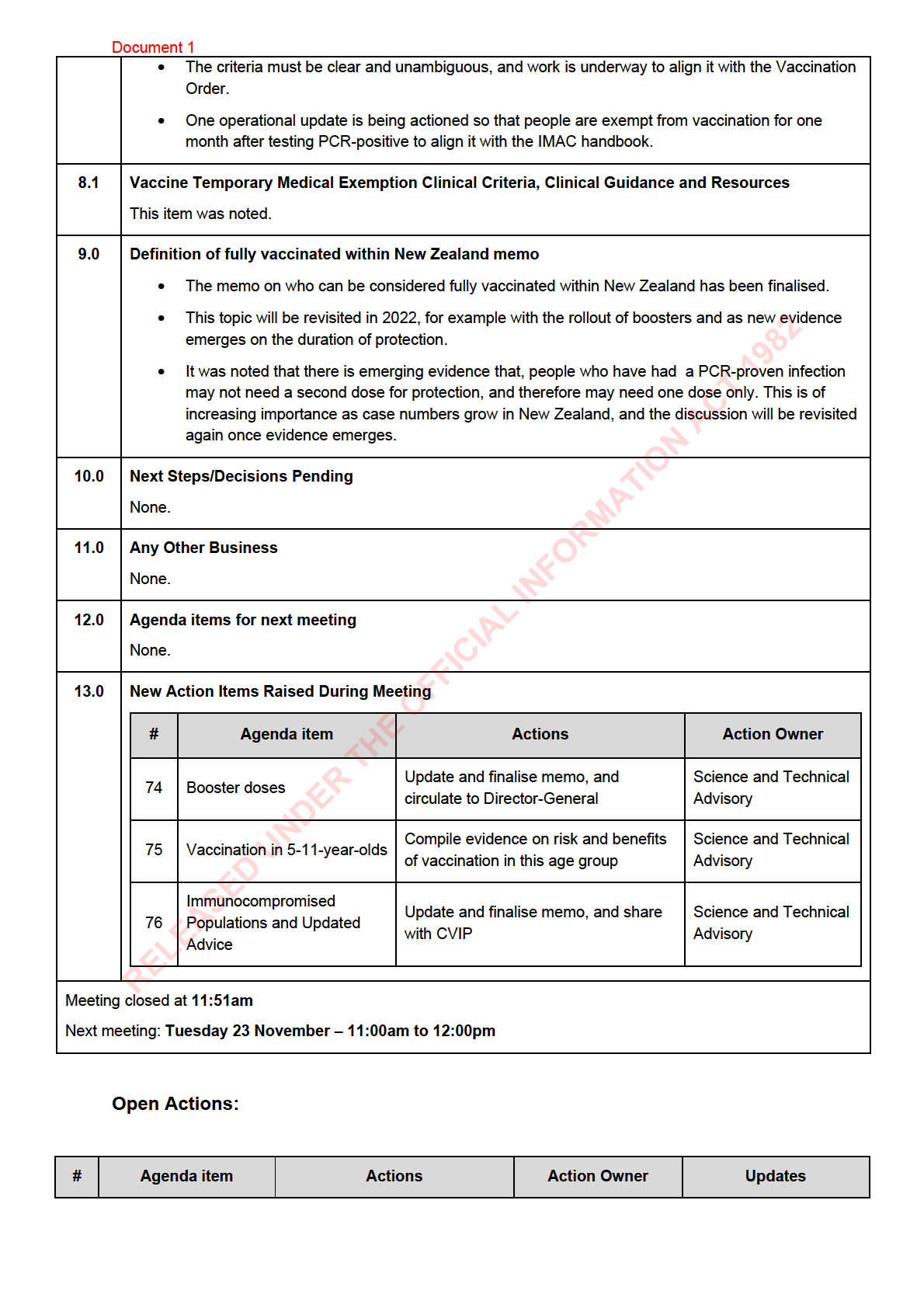
Document 1
Compile further evidence on the
Science and
49
Pfizer dosing error
link between dosing intervals
31/08 – Action raised
Technical Advisory
and reactogenicity.
Supporting Evidence
Finalise evidence brief and
Science and
64
for Healthcare Worker
19/10 – Action raised
share with CVIP and CV TAG
Technical Advisory
Vaccination Order
Review Pfizer’s application for
Decision to Use 5–11-
68
12-to-15-year-olds for evidence
Medsafe
19/10 – Action raised
Year-Olds
on dosages.
Immunocompromised
populations and
Revise memo with CV TAG’s
Science and
73
02/11 – Action raised
ATAGI’s update
feedback and share with CVIP
Technical Advisory
guidance
ACT 1982
Update and finalise memo, and
Science and
74
Booster doses
09/11 – Action raised
circulate to Director-General
Technical Advisory
Compile evidence on risk and
Vaccination in 5-11-
Science and
75
benefits of vaccination in this
09/11 – Action raised
year-olds
Technical Advisory
age group
Immunocompromised
Update and finalise memo, and
Science and
76
Populations and
09/11 – Action raised
share with CVIP
Technical Advisory
Updated Advice
INFORMATION
RELEASED UNDER THE OFFICIAL

Document 2
MINUTES: COVID-19 Vaccine Technical Advisory Group
Date:
Tuesday 23 November 2021
Time:
11:00am to 12:00pm
Location:
Teams: S9(2)(k)
Chair:
David Murdoch
Elizabeth Wilson, Ian Frazer, James Ussher, Nikki Moreland, Nikki Turner, Peter
Members:
McIntyre, Sue Crengle, Tony Walls
ACT 1982
Andi Shirtcliffe, Caroline McElnay, Chriselle Braganza, Daniel Bernal, Edwin
Ministry of Health Attendees:
Reynolds, Fiona Callaghan, Juliet Rumball-Smith N ki Stefanogiannis, Pippa
Scott, Imogen Roth, Mariana Traslosheros Reyes
Guests:
Hilary Longhurst
Ian Town, Brooke Hollingshead, Helen Petousis-Harris, Sean Hanna, John Tait,
Apologies:
Kris Golding
INFORMATION
1.0
Welcome and previous minutes
David Murdoch welcomed all Members and Attendees in his capacity as Deputy Chair of the COVID-19
Vaccine Technical Advisory Group (CV TAG).
Minutes of the last meeting (09 November 2021) were accepted.
2.0
Protection Framework
An update on the COVID 19 protection framework (CPF or ‘traffic light’ system) was given:
• CPF will come into force at 11.59 pm on Thursday 2nd December
• Can on y operate well in a highly vaccinated community, and implementation is dependent on
availability of vaccine certificates and proof of vaccination
• Factors to be considered under the settings for red, orange and green are:
o Vaccine coverage across population and equity of vaccine coverage
o Capacity of system to manage cases
RELEASED UNDER THE OFFICIAL
o Testing and contact tracing system capacity
o Transmission within the community
• This a process that is reviewed regularly
3.0
Update on Vaccine Rollout
An update was provided on the vaccine rollout:
Document 2
• AstraZeneca and boosters will be administered from 26 November, once we have the ability to
record them in the CIR
• Exemption process is working as planned, noting that most of the declines have been due to
incomplete applications
• Only eight DHBs are at less than 90% first doses, with most about to hit the 90% mark
• Advice on a third primary dose for immunocompromised patients has been finalised, noting that
the list is not exhaustive and provides scope for clinical judgement
4.0
Vaccine Certificates
An update was provided on vaccine certificates:
• Around 1.4 million people have received their Vaccine Pass
• The vaccination order will be updated by 29 November to include those vaccines which are on the
WHO EUL list (currently eight in total)
5.0
Vaccination in 5–11-year-olds
ACT 1982
The Science and Technical Advisory team provided an update on COVID-19 and vaccination in 5-11 year
old and discussion followed.
• Children are at a low risk of severe disease, although the risk is higher in some groups
• There is limited vaccine safety and efficacy data in this age group. With a reported 2 million plus
doses administered in this age group in the US, real world data is expected to help inform the
INFORMATION
advice
• Equity is an important factor in this group, and consideration will be given to prioritisation for
certain vulnerable groups
• Any future advice regarding vaccine certificates or mandates in this younger group, would need to
be considered separately to the advice on the decision to use.
6.0
Chronic Fatigue/ME and Vaccination
The Science and Technical Advisory (STA) team provided an overview of ME/CFS patients and
vaccination:
• STA is preparing a response to an external request that ME/CFS patients be exempt from
vaccination
• In addition, a literature review is being prepared externally by the requestor around the evidence
for this
• The current status for these patients is that exemptions can be granted if there is an adverse
event after the first dose
RELEASED UNDER THE OFFICIAL
• There is concern that exemptions in this group could provide precedence for other groups
• STA will continue to assess the evidence and bring back to CV TAG for discussion
7.0
Update from BMI Needle Length Study
A brief update was included in the agenda for noting.
8.0
Next Steps/Decisions Pending


Document 3
MINUTES: COVID-19 Vaccine Technical Advisory Group
Date:
Tuesday 30 November 2021
Time:
11:00am to 12:00pm
Location:
Teams: S9(2)(k)
Chair:
Ian Town
Elizabeth Wilson, Helen Petousis-Harris, Ian Frazer, James Ussher, Nikki
Members:
Moreland, Nikki Turner, Peter McIntyre, Sean Hanna, Tony Walls
ACT 1982
Brooke Hollingshead, Caroline McElnay, Chriselle Braganza, Daniel Bernal,
Ministry of Health Attendees:
Edwin Reynolds, Fiona Callaghan, Imogen Roth, Ju iet Rumball-Smith, Mariana
Traslosheros Reyes, Niki Stefanogiannis, Pippa Scott
Guests:
Hilary Longhurst
Apologies:
David Murdoch, John Tait, Kris Golding, Andi Shirtcliffe, Sue Crengle
INFORMATION
1.0
Welcome and previous minutes
Ian Town welcomed all Members and Attendees in his capacity as Chair of the COVID-19 Vaccine
Technical Advisory Group (CV TAG) and provided an overview of the vaccination program progress.
Minutes of the last meeting (23 November 2021) were accepted.
A request was made for further discussion in the minutes on one dose in 12–15-year-olds. This is covered
further under any other business, below.
2.0
Pfizer Vaccination in 5-11 year-olds
The Science and Technical Advisory team provided an update on COVID-19 and vaccination in 5-11 year
olds and discussion followed:
• There is concern that if implemented this will need to be very carefully considered in the context of
our current national immunisation schedule, particularly with respect to equity of delivery of all
childhood vaccines.
• Noted that including numbers needed to treat to prevent disease and death in children and in 5-11
year olds would be valuable, noting this is a commonly used statistic
RELEASED UNDER THE OFFICIAL
• Noted that children at high risk of severe illness should be prioritised for COVID-19 vaccines
• Identified that a longer than three week interval between doses would be preferable
• STA will continue to assess the evidence and bring it back to CV TAG for discussion
3.0
Modelling data and assumptions
The Science and Technical Advisory team provided an update on modelling data and assumptions and
discussion followed:
Document 3
• CV TAG asked to be provided with a list of the model assumptions and parameter values
4.0
Vaccine Certificates
No update.
5.0
Vaccination after previous SARS-CoV-2 infection (primary and booster schedules)
The Science and Technical Advisory team provided an update on vaccination after previous SARS-CoV-2
infection:
• Noted this was a current issue and required a fair level of detail when decision making for
clinicians
• Noted that any decisions around pregnancy need to be high priority
• Urgent advice will be provided for clinicians on vaccination after SARS-CoV-2 infection and
possible temporary exemptions in the light of vaccine mandates.
6.0
Update of fully vaccinated definition to include boosters
ACT 1982
The Science and Technical Advisory team provided an update on the definition of fully vaccinated and the
inclusion of boosters in the definition:
• Noted that this would be a comprehensive piece of work that is being raised early in the booster
program and the definition of fully vaccinated
• STA will continue to assess the evidence and bring it back to CV TAG for discussion
INFORMATION
7.0
ME/CFS and COVID-19 Vaccination
An update was provided on the request for ME/CFS patients to be exempt from vaccination:
• Advised that we need to distinguish between the clinical issues and the scientific issues
• Consideration could be given to ME/CFS patients receiving a lower dose of the Pfizer vaccine for
those patients with chronic and debilitating disease symptoms
• Noted that it is important that patient groups feel heard, and there is a potential opportunity for a
clinical trial which will address these issues
• STA will continue to assess the evidence and bring it back to CV TAG for discussion
8.0
Myocarditis research sub-TAG
An update was provided on the sub-TAG meeting
• An in tial discussion was had at the sub-TAG meeting and future topics were discussed, including
detection and monitoring of milder cases of myocarditis
• There will be another meeting to follow up and prepare some specific research proposals.
RELEASED UNDER THE OFFICIAL
9.0
Final Memo: Booster Vaccinations
Noted
10.0
Final Memo: Decision to Use AZ
Noted
11.0
Next Steps/Decisions Pending
None noted

Document 3
Any other business –
Draft a memo outlining CV
issue of requiring two
Science and
78
TAGs position on requiring two
30/11 – Action raised
doses of vaccine for
Technical Advisory
doses for under 18s
12-15 year olds
ACT 1982
INFORMATION
RELEASED UNDER THE OFFICIAL

Document 4
MINUTES: COVID-19 Vaccine Technical Advisory Group
Date:
Tuesday 07 December 2021
Time:
11:00am to 12:30pm
Location:
Teams: S9(2)(k)
Chair:
Ian Town
Elizabeth Wilson, Helen Petousis-Harris, Ian Frazer, James Ussher, Nikki
Members:
Moreland, Nikki Turner, Peter McIntyre, Sean Hanna, Sue Crengle, Tony Walls
ACT 1982
Andi Shirtcliffe, Brooke Hollingshead, Caroline McElnay, Daniel Bernal, Edwin
Ministry of Health Attendees:
Reynolds, Fiona Callaghan, Imogen Roth, Juliet Rumball-Smith, Mariana
Traslosheros Reyes, Pippa Scott
John Tait, Kris Golding, Jin Russell, Danny de Lore, Erik Andersen, Owen
Guests:
Sinclair, Teuila Percival, Marise Stuart, Andrew Simpson, Liam McConnell
Apologies:
David Murdoch, Chriselle Braganza, Niki Stefanogiannis
INFORMATION
1.0
Welcome and Previous Minutes
Ian Town welcomed all Members and Attendees and Guests in his capacity as Chair of the COVID-19
Vaccine Technical Advisory Group (CV TAG).
Minutes of the last meeting (30 November 2021) were accepted.
2.0
Modelling Data and Assumptions
Update deferred
3.0
Vaccination After Previous SARS-CoV-2 Infection
As part of the discussion under 7.0, below, CV TAG members agreed that a 3-month class exemption
after PCR confirmed infection could resolve current issues around wording of exemption after infection
(currently states until complete recovery), providing sufficient time for full vaccination to be completed.
4.0
Two Doses of Vaccine for Under 18s
An update was provided on the memo advising that those aged under 18 should not be required to have
RELEASED UNDER THE OFFICIAL
two doses of vaccine under vaccine mandates
• CV TAG does not want to see two doses of vaccine absolutely required for under 18s to be able to
work
• STA outlined the current status of the draft memo, aimed at clarifying the CV TAG advice that
vaccine mandates should not apply to those under 18
• Policy, health legal and crown law will continue working with STA and CV TAG
Document 4
• STA will continue to work with colleagues towards making vaccination requirements for those
under 18 clearer.
5.0
Pfizer Vaccination in 5-11 year-olds
Guests were welcomed by the Chair and provided an overview of their affidavit to the Waitangi Tribunal
about the expected impacts to tamariki Māori and their whānau with the planned shift to the COVID-19
protection framework.
An update was provided on vaccination in 5-11 year olds and discussion followed:
• STA outlined the timeline for decision to use for 5-11 year olds and advised Cabinet will make
their decision on 23 December, pending Medsafe approval
• The contribution to the Waitangi Tribunal claim was outlined, noting that tamariki Māori do not
always have their views represented due to the small numbers of experts and advocates involved
in decision making
• It was noted that if decisions were made for the majority or the average but tamariki Māori were
ACT 1982
not considered then the health inequities that already exist will be exacerbated
• It was noted that tamariki Māori do not have the same standard of health as other children, as they
bear the burden more heavily with co-morbidities, which are linked with poorer outcomes as a
result of SARS-CoV-2 infection
• The Māori population is younger and has on average more tamariki in an average household
• Tamariki Māori have a right to an intervention that protects them against a direct harm from a
INFORMATION
preventable disease, and the right to participate in protecting the people around them
• It was felt that if we do not make the vaccine available we will having rolling outbreaks in tamariki
Māori, resulting in isolation, sick caregivers, and whānau in hospital
• The te ao Māori understanding of tamariki as being a part of a whānau and community was
highlighted, rather than solely as an individual with only individual benefit
• Additional points were raised from the literature indicating that non-white children are likely to
disproportionately be affected by COVID-19 with respect to MIS-C, as well as the loss of a
parent/caregiver
• It was outlined that including 5-11 year olds in the vaccination program can strengthen efforts to
immunise older Māori people - noting whānau will get vaccinated together
• Operational suggestions for the rollout were discussed, and this will be taken to the
implementation group with regards to the national immunisation programme
• Delivery of successful vaccinations for 5-11 year olds need to be with Māori health providers and
networks and iwi and hapu
• The suggestion of a Māori paediatrician(s) joining CV TAG as a continuing member was made,
RELEASED UNDER THE OFFICIAL
and supported by the group
• STA will develop a draft memo with recommendations for CV TAG to consider next week
Guests were thanked by the Chair and left the meeting
ACTION: CV TAG chair to consider co-opting additional members to CV TAG for 2022
6.0
Next Steps/Decisions Pending
None noted

Document 4
Share modelling data and
Science and
77
Any Other Business
23/11 - Action raised
assumptions with CV TAG
Technical Advisory
Any other business –
Draft a memo outlining CV
issue of requiring two
Science and
78
TAGs position on requiring two
30/11 – Action raised
doses of vaccine for
Technical Advisory
doses for under 18s
12-15 year-olds
Consider a Māori
Pfizer Vaccination in 5-
79
paediatrician/s to become a
Chair
07/12 – Action raised
11 year-olds
standing member of CV TAG
ACT 1982
INFORMATION
RELEASED UNDER THE OFFICIAL

Document 5
MINUTES: COVID-19 Vaccine Technical Advisory Group
Date:
Thursday 20 January 2022
Time:
11:00am to 12:30pm
Location:
S9(2)(k)
Chair:
Ian Town
Danny de Lore, David Murdoch, Elizabeth Wilson, Helen Petousis Harris, James
Members:
Ussher, Nikki Moreland, Nikki Turner, Owen Sinclair, Peter McIntyre
Andi Shirtcliffe, Brooke Hollingshead, Daniel Bernal, Fiona Callaghan, Juliet
ACT 1982
Ministry of Health Attendees:
Rumball-Smith, Mariana Traslosheros Reyes, Pippa Scott
Guests:
John Tait, Karin Van Bart, Frances Graham Phoebe Currie
Caroline McElnay, Chriselle Braganza, Edwin Reynolds, Ian Frazer, Imogen
Apologies:
Roth, Niki Stefanogiannis, Sean Hanna, Sue Crengle, Tony Walls
INFORMATION
1.0
Welcome and Previous Minutes
Ian Town welcomed all Members and Attendees and Guests in his capacity as Chair of the COVID-19
Vaccine Technical Advisory Group (CV TAG).
Minutes of the last meeting (07 December 2021) were accepted subject to the following changes to Item
2.0 CV TAG Draft Recommendations on Vaccination in 5-11-year-olds
• The previous minutes note
It was felt very strongly that CV TAG do not want vaccines mandated
(formally or informally) in this age group and ensuring there are no unintended consequences for
children if they are not vaccinated, even as a matter of choice, due to the lack of clear benefit for
the child. The “lack of clear benefit” was in relation to mandates and not vaccines and this should
be clarified in revised minutes.
2.0
Revisit Discussions:
2.1 Interval between doses for <30s
Discussion point: Does the interval between doses need to be reconsidered and lengthened in light of
the risk of myocarditis?
• Continued concern was expressed about the risk of myocarditis for those aged under 30. While it
RELEASED UNDER THE OFFICIAL
was noted that evidence is limited on the ways to reduce myocarditis, initial data from Ontario in
Canada suggests a wider interval between doses reduces the risk of myocarditis.
• Based on first principles of immunology, a three-week interval between doses was not seen as
typical.
• Any messaging on a change to suggested schedules would need to be based on the benefits in
the immune response, due to not wanting to have unintended consequences on vaccine
acceptance. It was discussed that the vaccine rollout among 5-11-year-olds was framed as an
8-week interval, with the option to have it sooner, and this could also be more broadly applied.
Document 5
• It was agreed that a brief update to the advice will be issued with the new data with from Ontario
and discussed with the Director-General
2.2 Vaccine certificates for under 18s
Discussion point: Is this clinically appropriate and aligned with advice for 5-11-year-olds? What steps
have been taken from Policy?
• Concern was expressed that COVID-19 vaccine certificates (CVCs) for those aged under 18
might seek two adult doses three-weeks apart when clinically another option may be better, and
concern was also expressed about the impact that restrictions would have on this group when
vaccines provide good protection.
• There is a need for flexible guidance on what is considered well protected and of clinical benefit
to the individual, with variations by age, clinical considerations, history of infection etc These
guidelines could allow for longer intervals and lower (paediatric) second doses rather than a
rigid framework.
• It was noted that this would have implications for CVCs and mandatory vaccine orders that need
ACT 1982
to be worked through and is in line with the broader National Immunisation Schedule.
• A formal policy statement is needed to ensure CVCs are not used among 5-11-year-olds due to
concern of the impact of social restrictions.
• CVIP Clinical with STA support will develop a framework for CV TAG’s consideration and
endorsement in conjunction with Policy.
INFORMATION
2.3 Myocarditis post-vaccine
Discussion point: Should individuals who had myocarditis after their first Pfizer dose be recommended an
exemption or AstraZeneca?
• Guidance should be balanced to ensure people are not unfairly assigned to social restrictions.
• It was noted that there is some evidence of a risk of myocarditis with the AstraZeneca dose too.
• The risk of myocarditis from infection may be greater for most.
• Evidence collation is required on the safety of AstraZeneca and Janssen given as a second
dose. STA to collate an RfA at pace on the latest evidence.
2.4 Previous infection
Discussion point: Is there evidence that infection provides similar protection to one dose, or is a second
primary dose needed?
• It was noted that this was relevant to three groups
RELEASED UNDER THE OFFICIAL
o Young people with disease (who will now be covered by changes in the exemption
criteria)
o Whether young people with proven disease need two primary doses
o Whether young people with proven disease and two primary doses need a booster,
some of whom will be captured under mandatory vaccine orders.
• Consideration of the efficacies, immune response and protection provided by each of these
experiences is required and would be captured under the framework being developed under item
2.2.
Document 5
• A quick review of the advice given by other peak bodies (e.g., ATAGI, JCVI, MHRA, ACIP) is
required to see what other jurisdictions are recommending.
2.5 Alternative schedule: Paediatric dose after adult dose
Discussion point: For people who have an adverse reaction to Pfizer post-first dose (e.g., adolescents
aged 12-15 or adults with unclear aetiology e.g., severe CFS/ME response), could the lower dose (10
mcg) formulation be an option, and does off-label use cover it?
• Concerns were raised regarding administrative errors (e.g., between the adult and paediatric
doses, expired doses etc.) being given. These will be dealt with through clinical quality
assurance processes.
• It was noted that 12-15-year-olds who may benefit from having the lower paediatric dose could
be given this off-label at clinical discretion. There is evidence they are protected well by this
dose.
• People with ME/CFS who have experienced exacerbated conditions that may be linked to the
vaccine could also be offered the paediatric formulation. A protocol for this will be developed by
ACT 1982
CVIP Clinical, relevant clinicians, and some CV TAG members.
• It was raised that a formal uncoupling of science and clinical advice may be needed from the
policy and legislative frameworks for CV TAG recommendations.
• A discussion is required with the Ministry of Health teams managing CVCs/Data and Digital to
ensure this would still meet requirements for CVCs and be easy to implement
3.0
mRNA Injections and Aspiration
INFORMATION
• Queries on the benefits of aspiration continue to be received.
• IMAC have a formal statement discussing the issue, and the University of Auckland has a blog,
and queries should be directed to these sources.
• A further statement is required that having some blood in the needle can occur.
4.0
Booster Interval Final Memo
• It was noted that the Ministry of Health Policy team, on guidance from the Director-General,
recommended that the interval be changed to 4 months (rather than the 5 months in CV TAG’s
advice).
• Guidance for pregnant and immunocompromised people was updated at pace to be four months
with ATAGI updating their guidance, and via consultation with some CV TAG members, with a
note hat further evidence and recommendations would be sought from CV TAG.
• A further discussion and memo are required to formalise the advice on boosters in pregnant
people, and boosters (fourth doses) in the immunocompromised.
• There may be a role for serology for immunocompromised people in case of exposure to
RELEASED UNDER THE OFFICIAL
measure antibody response and develop a management plan if this response is not strong.
Clinicians may consider antibody tests at clinical discretion.
5.0
Myocarditis Research Project Update
• This research will be following up with people who have had myocarditis or pericarditis after their
vaccination, and their healthcare providers. There are estimated to be 200-300 people eligible.
CBG Health have been contracted to run the survey. Ethics application are being submitted this
week, and the study will be starting mid-late-February.
• This research will be put in touch with the research underway at the University of Auckland.
Document 5
6.0
Updated Exemptions
• Updates made to the exemptions criteria have gone through which gives more freedom for to
operate, however the panel is open to further feedback on criteria.
• 2.a currently says ‘attributed to previous vaccine’ however this needs to be changed to ‘causally
associated’.
7.0
Update on Mandated Boosters
This item was noted.
8.0
Decision to Use for 5–11-Year-Olds Final Memo
This item was noted.
9.0
Rollout Data on Myocarditis in 5–11-year-olds
This item was noted.
ACT 1982
10.0
CV TAG work for 2022
• Heterologous schedules for boosting
• 4th boosters (for immunocompromised/ all)
• Definition of fully vaccinated (and boosters)
• Second dose for 5–11-year-olds – full safety review required in early February
• Boosters for 12-15-year-olds and 5-11-year-olds
INFORMATION
o It was noted that initial caution is required that the use of boosters in this population is
not a certainty, and currently there is a lack of evidence for their need.
• Roles of Janssen and AstraZeneca in the rollout
• Novavax decision to use
• Moderna decision to use
• Vaccinating 2–5-year-olds
11.0
Next Steps/Decisions Pending
None.
12.0
Any Other Business
Secretariat business
• The regular meeting time may need to be moved to 10.30am Tuesdays, however availability
from CV TAG members will be sought, and this would not be for the next two weeks.
RELEASED UNDER THE OFFICIAL
• CV TAG members can expect to receive a work programme for the year noting when items will
likely be discussed, and what CV TAG’s role wil be.
• All finalised and signed off CV TAG memos are in the process of being uploaded to the Ministry
of Health website for public access, pending approval.
13.0
Agenda Items for Next Meeting
Heterologous schedules for boosting
4th doses for the immunocompromised

Document 5
Develop clinical guidance
Vaccine certificates
framework on what is
81
for under 18s
considered sufficient protection CVIP Clinical
20/01 – Action raised
Previous infection
for CV TAG’s consideration
and endorsement
Develop formal policy
Vaccine certificates
statement that CVCs should
82
STA
20/01 – Action raised
for under 18s
not be used among 5-11-year-
olds
Collate evidence on risk of
Myocarditis post-
83
myocarditis post-vaccine with
STA
20/01 – Action raised
vaccine
AstraZeneca and Janssen
Collate advice from peak
84
Previous infection
bodies on immune response
STA
20/01 – Action raised
and vaccine recommendations.
Protocol to be developed to
ACT 1982
Alternative schedule:
ensure access to paediatric
CVIP Clinical with
85
Paediatric dose after
doses of the vaccine are
20/01 – Action raised
support from STA
adult dose
available to those who may
benefit from it.
Alternative schedule:
Advice to be sought on the
86
Paediatric dose after
STA
20/01 – Action raised
impact on CVCs
adult dose
INFORMATION
Send updated comment for
mRNA Injections and
87
statement to include comment
STA
20/01 – Action raised
Aspiration
on blood and myocarditis
Develop recommendation and
Booster Interval Final
write memo on booster interval
88
STA
20/01 – Action raised
Memo
for pregnant and
immunocompromised people
89
Updated exemptions
Update language of 2.a criteria
STA
20/01 – Action raised
RELEASED UNDER THE OFFICIAL










