



133 Molesworth Street
PO Box 5013
Wellington 6140
New Zealand
T+64 4 496 2000
19 April 2022
Heather Darby
By email
[FYI request #18947 email]
Ref
H202204257
Tēnā koe Heather
Response to your request for official information
Thank you for your request under the Official Information Act 1982 (the Act) to the Ministry of
Health (the Ministry) on 22 March 2022 for information regarding COVID-19 vaccines. I will
respond to each part of your request in turn.
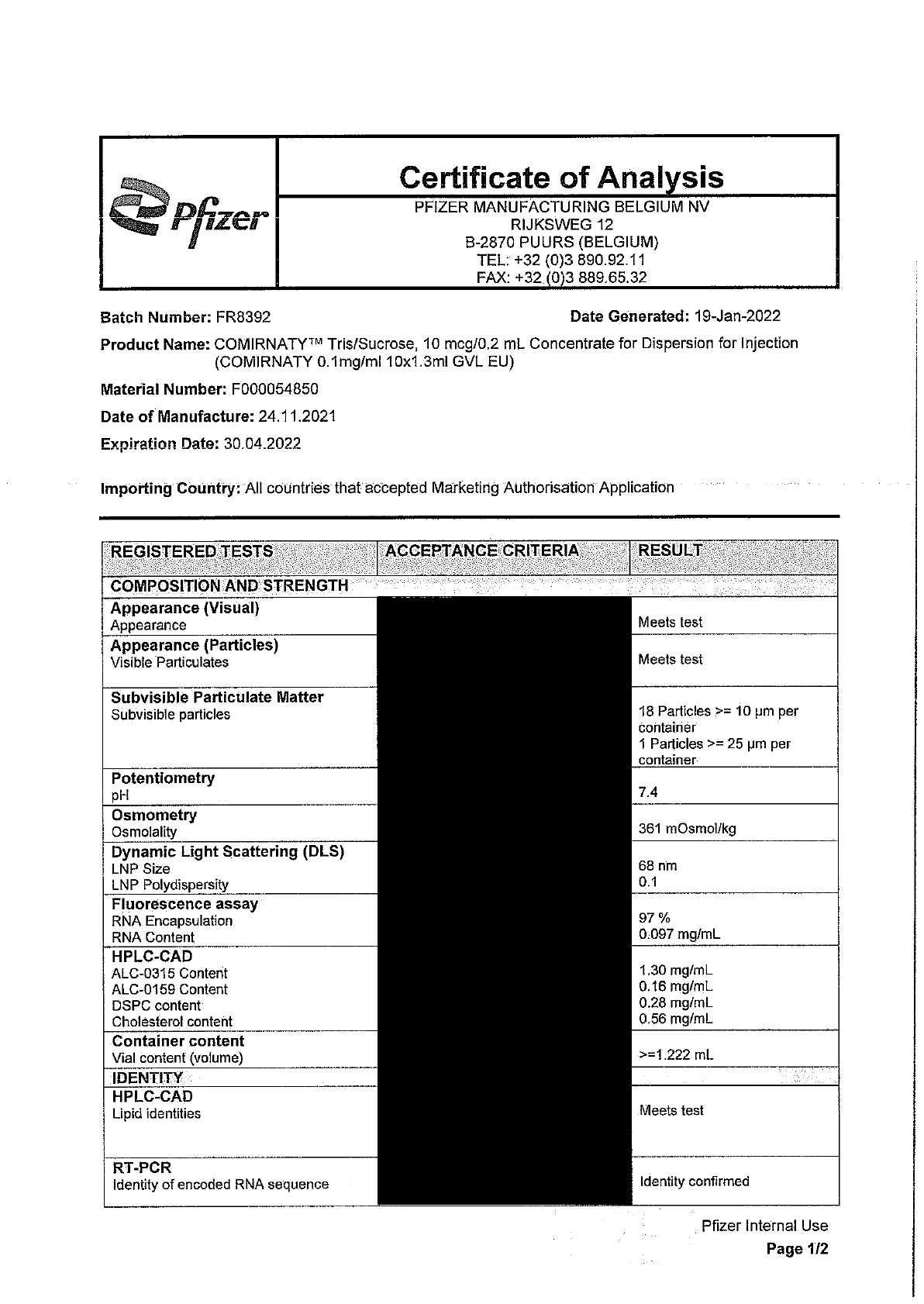
1. Certificates of Analysis for the first three batches of vaccine distributed in New
Zealand.
Please find copies of these documents enclosed with some information withheld under the
following sections of the Act:
• 9(2)(a) to protect the privacy of natural persons; and
• 9(2)(b)(ii) as its release would likely unreasonably prejudice the commercial position of
the person who supplied the information; and
• 9(2)(ba)(ii) to protect information that is subject to an obligation of confidence in which
making it available would likely damage the public interest.
Where information is withheld, this is noted in the document itself. I have considered the
countervailing public interest in release in making this decision and consider that it does not
outweigh the need to withhold at this time.
2. Independent batch certification, such as UK National Institute for Biological
Standards and Control (NIBSC) certification, EU Official Control Authority Batch
Release (OCABR) certification, Australian TGA batch release assessment for all
batches distributed in New Zealand.
These documents are withheld in full under section 6(b)(ii) of the Act, as its release would
prejudice information entrusted to the Government of New Zealand on a basis of confidence by
any international organisation.
3. Data to further characterise the truncated and modified mRNA species present in the
finished product which addresses results from ion pairing RP-HPLC addressing
5’cap levels and presence of the poly(A) tail and addresses the potential for
translation into truncated S1S2 proteins/peptides or other proteins/peptides.
4. Relevant protein/peptide characterisation data for predominant species.
5. Evaluation of any homology between translated proteins (other than the intended
spike protein) and human proteins that may, due to molecular mimicry, potentially
cause an autoimmune process.
6. Analysis of the main peak of the RNA integrity test representing the full-length RNA
that addresses 5’cap levels and presence of the poly(A) tail.
7. Reassessment of the active substance specification for the DNA template purity and
impurities.
8. Active substance process validation data regarding the finalised indirect filter
qualification assessment and the shipping validation between sites.
9. The capability of the next generation sequencing technology platform to detect lower
amounts of RNA species of alternative sequence in the presence of the correct,
more abundant RNA for the active substance.
10. The results and the assay suitability for the cell-based flow cytometry and the
western blot method used for biological characterisation of protein expression for the
active substance.
11. A summary of the validation/verification status of the immunoblot analytical
procedure used to detect double stranded RNA (dsRNA) in the active substance.
12. Data comprising batch analyses of a suitable number of commercial batches as well
as analyses of batches that have been used in the (ongoing) clinical trials.
13. Specifications and results of introducing an active substance to control poly(A) tail
length and how it was controlled on each batch.
14. Data to support the suitability of the method used for %poly(A) tail in Q14 [Q13]
above.
15. Revised specifications of the mRNA integrity and polydispersity finished product.
16. Data to support the suitability of the method used for potency determination.
17. The finished product acceptance criteria for potency.
18. Control strategy assessment results for Lipid-related impurities.
19. The risk assessment with respect to the potential presence of elemental impurities in
the active product based on the general principles outlined in Section 5.1 of ICH
Q3D and Ph. Eur. monograph Pharmaceutical Preparations (2619).
20. Process development for ALC-0315 with emphasis on the identification and purge of
impurities.
21. Specified impurities for ALC-0315 and appropriate specification limits for individual
impurities.
22. Acceptance criteria for specified and un-specified impurities for ALC-0315.
23. Details about how the solvent residues that are used in the manufacture of the ALC-
0315 excipient are controlled.
24. The ALC-0315 assay and impurities limits.
25. Method validation reports for assay, impurities, and residual solvents for ALC-0315.
26. ALC-0315 impurity standard information for any identified impurities reported.
27. The impact of the molecular weight and polydispersity of carboxy-MPEG on ALC-
0159, including acceptance criteria, for these parameters in the starting material.
28. Reports on the duration of efficacy and the requirement for booster doses.
This information is withheld in full under section 9(2)(b)(ii) of the Act where its release would
likely unreasonably prejudice the commercial position of the person who supplied the
information. Please note, I have considered the countervailing public interest in release in
making this decision and consider that it does not outweigh the need to withhold at this time.
Information on the duration of efficacy of the Pfizer Comirnaty vaccine is available in the data
sheet published at:
www.medsafe.govt.nz/profs/Datasheet/c/comirnatyinj.pdf.
Page 2 of 4

The Ministry is continuing to monitor international research and developments surrounding
COVID-19 variants and COVID-19 vaccines. The most recent variants update includes a
summary of vaccine effectiveness against the Omicron variant and can be found at:
www.health.govt.nz/system/files/documents/pages/variants_update_-_omicron_-
_25_march_2022.pdf. The Ministry also regularly updates the Science News page for up to date information regarding
COVID-19 and the vaccine:
www.health.govt.nz/covid-19-novel-coronavirus/covid-19-resources-
and-tools/covid-19-science-news. More information about booster doses is available at
www.covid19.govt.nz/covid-19-vaccines/how-to-get-a-covid-19-vaccination/getting-your-
booster-dose/.
29. Reports on efficacy including asymptomatic infection in the vaccinated group,
vaccine failure, immunogenicity, and efficacy in population subgroups.
Clinical study reports provided to Medsafe by Pfizer are withheld in full under section 9(2)(b)(ii)
of the Act.
30.
The final Clinical Study Reports for Study C4591001 and Study BNT162-01.
This information is refused under section 18(g)(i) as the information requested is not held by the
Ministry and there are no grounds for believing it is held by another agency subject to the Act.
31. The latest Safety Update Report.
Safety reports for the COVID-19 vaccine are published on the Medsafe website at:
www.medsafe.govt.nz/COVID-19/vaccine-report-overview.asp. The publication date for the next
report is also available at this link. Therefore, this part of your request is refused under section
18(d) as the information requested is publicly available.
Under section 28(3) of the Act, you have the right to ask the Ombudsman to review any
decisions made under this request. The Ombudsman may be contacted by email at:
[email address] or by calling 0800 802 602.
Please note that this response, with your personal details removed, may be published on the
Ministry website at:
www.health.govt.nz/about-ministry/information-releases/responses-official-
information-act-requests. Nāku noa, nā
Jan Torres
Acting Manager, OIA Services Office of the Director-General
Page 3 of 4
Appendix 1: List of documents for release
#
Date
Document details
Decision on release
1
20 December 2021
Certificate of Analysis for the Released with some information
Comirnaty (Pfizer) COVID-19 withheld under the following
Vaccine.
sections of the Act:
• section 9(2)(a) to protect
the privacy of natural
persons;
• 9(2)(b)(ii) as its release
would likely unreasonably
prejudice the commercial
position of the person who
supplied the information;
and
• section 9(2)(ba)(ii) to
protect information that is
subject to an obligation of
confidence in which making
it available would likely
damage the public interest.
2
19 January 2022
Page 4 of 4

Document 1
1982
THE
ACT
S9(2)(ba)(ii)
UNDER
INFORMATION
RELEASED
OFFICIAL

Document 1
1982
THE
ACT
UNDER
INFORMATION
RELEASED
OFFICIAL
Document 2
1982
THE
ACT
S9(2)(ba)(ii)
UNDER
INFORMATION
RELEASED
OFFICIAL

Document 2
1982
THE
ACT
UNDER
INFORMATION
RELEASED
OFFICIAL








