 Memorandum
COVID-19 Vaccine and Immunisation Programme: Gantt Chart &
Memorandum
COVID-19 Vaccine and Immunisation Programme: Gantt Chart &
Released
Governance Structure
Date due to MO: 15 October 2020
Action required by:
N/A
Security level:
IN CONFIDENCE
Health Report number: 20201852
To:
Hon Chris Hipkins, Minister of Health
under the Official Information Act 1982
Copy to:
Hon Julie Anne Genter, Associate Minister of Health
Contact for telephone discussion
Name
Position
Telephone
Dr Ashley Bloomfield
Director-General of Health
9(2)(a)
Mathew Parr
Programme Director
9(2)(a)
Action for Private Secretaries
N/A



 COVID-19 Vaccine and Immunisation
COVID-19 Vaccine and Immunisation
Programme: Gantt Chart & Governance
Released
Structure
Purpose of report
1.
This report responds to your request for a Gantt Chart that outlines the key milestones
for the COVID-19 Vaccine & Immunisation Programme (Programme). It also provides
you with a view of the governance structure for the Programme. This covers the
under the Official Information Act 1982
fol owing workstreams:
Vaccine Purchasing Workstream
COVID-19 Immunisation Implementation Programme
National Immunisation Solution (NIS).
Background
2.
We have developed an Immunisation Programme that can promptly rol out the vaccine
once it arrives in New Zealand. Several workstreams are progressing at pace and in
paral el to ensure tight timeframes are met. Senior internal governance for the
Programme is in place, and a new external Governance Group is being established to
support and provide assurance on delivery, with representation across key sectors.
Gantt chart
3.
At your request, a Gantt Chart is attached at Appendix A that provides details about the
workstreams involved in purchasing and immunising for the Pfizer vaccine, which has
been announced, and the key milestones. Draft plans for further vaccines are included
and the plan wil evolve further as additional vaccines are confirmed which wil further
impact on timelines.
4.
An underlying assumption is that the Pfizer vaccine wil arrive in New Zealand on 1
January 2021, noting that these timelines are likely to flex dependent on vaccine
availability. Officials are planning ahead of this date to ensure we are ready to deliver in
the event it lands earlier than anticipated. The latest delivery schedule received from
Pfizer suggests the January deadline is highly unlikely. At present, Medsafe has not
received information from Pfizer to start the regulatory approval process.
5.
A critical factor for the delivering the vaccine is to ensure the National Immunisation
Solution (NIS) is developed by 1 January, with an improved product ready later in Q1
2021. This IT system is essential to the rol out of a vaccine as it wil be essential for
capturing population information.
6.
Work is occurring in paral el and there are a number of streams which are
interdependent on each other. The critical path milestones are mapped out at
Appendix A and include the fol owing:
Health Report: 20201852
2















Cold storage and consumable requirements and funding
Standing up ultra-low temperature storage and supply chain
Securing and training the required workforce
Medsafe approval
Released
Ministerial decision to use and commence delivery.
7.
To ensure there is a contingency option available by 1 January 2021 an interim solution
is being developed for the NIS. This wil be built in paral el with the final product should
the vaccine arrive prior to the anticipated delivery date. As more certainty emergences
on the delivery date, this interim arrangement may be stood down. The 1 January 2021
timeframe would present considerable risk in the delivery of a successful programme.
However, programme planning has commenced to al ow for this possibility, while stil
under the Official Information Act 1982
building a robust and credible programme that is aligned to more likely delivery dates.
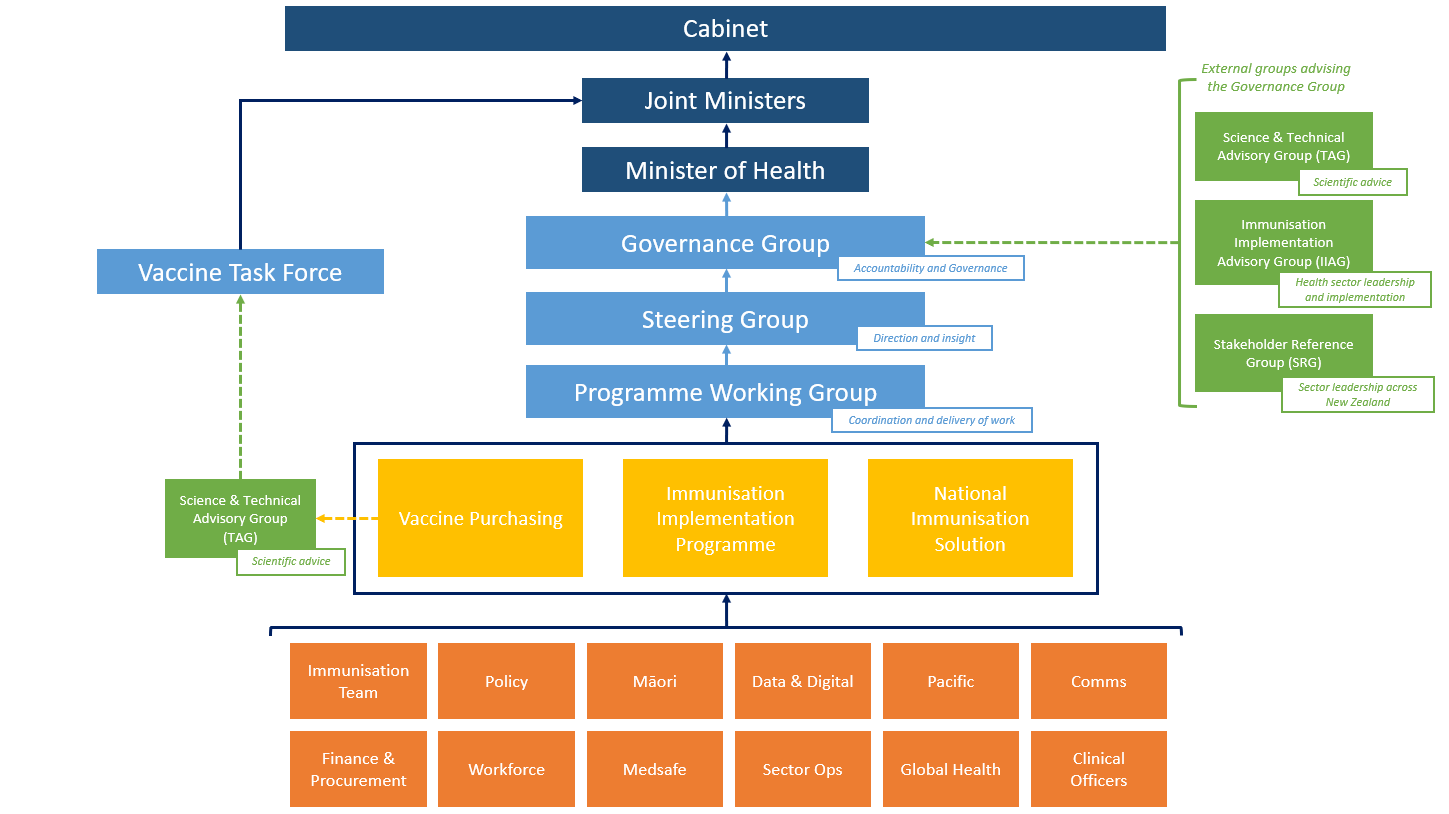
Governance structure
8.
In a fluid environment with highly changeable subject matter such as COVID-19, it is
important to respond in an agile way to competing demands. This is a high profile and
significant work programme, meaning careful management and governance is required
in order to achieve tight timeframes.
9.
To support the delivery of the Programme in line with the timelines set out in the Gantt
Chart, a governance structure has been strengthened and is outlined at Appendix B.
This structure shows the formal lines of accountability between each layer.
10.
A Governance Group is being established that wil act as a strategic decision-maker for
the overal delivery of the Programme. It wil meet on a fortnightly basis and wil report
directly to you. Membership of the Governance Group wil include:
Chair: Dame Dr Karen Poutasi, Commissioner, Waikato District Health Board
Dr Ashley Bloomfield, Director-General of Health
Steve Maharey, Chair of PHARMAC
Bruce Plested, Chairman of Mainfreight
Carolyn Tremain, Chief Executive, MBIE
Ngāhiwi Tomoana, Chair of the Māori Economic Development Panel and Chair of
Ngāti Kahungunu
Murray Jack, IT expert and former CEO and Chair of Deloitte
Dr Fa’afetai Sopoaga, Associate Professor, University of Otago
John Whaanga, Deupty Director-General Māori Health, Ministry of Health.
11.
The Governance Group wil maintain a connection with the Vaccine Taskforce, which is
led by MBIE and chaired by Dr Peter Crabtree, and this important connection is
recognised by the inclusion of Carolyn Tremain.
Health Report: 20201852
3
 Next steps
Next steps
12.
Work on the Programme wil continue at pace, including establishing the Governance
Group and other advisory functions who wil provide advice.
13.
Officials wil continue to update you weekly about the progress of the Programme.
Released
14.
We wil continue to work closely with our communications counterparts at MBIE to
ensure there is consistent messaging between agencies and that we are telling an
aligned story about both vaccine procurement and the overal immunisation plan.
under the Official Information Act 1982
Jane Kelley
Acting Deputy Chief Executive
COVID-19 Health System Response
Health Report: 20201852
4







Released
Appendix A – Gantt Chart
under the Official Information Act 1982
Health Report: 20201852
5

Released
Appendix B – Governance Structure
under the Official Information Act 1982
Health Report: 20201852
6
Document Outline





























