
133 Molesworth Street
PO Box 5013
Wellington 6140
New Zealand
T+64 4 496 2000
3 May 2023
J Bruning
By email: [FYI request #22343 email]
Ref:
H2023022968
Tēnā koe
Response to your request for official information
Thank you for your request under the Official Information Act 1982 (the Act) to Manatū Hauora
(the Ministry of Health) on 31 March 2023 for information regarding COVID-19 and pregnancy.
You requested:
Q(1) Please provide the dates and titles of all risk/benefit analyses undertaken which
reviewed the data/science of risks and benefits to the pregnant mother and fetus for the
primary course of injection of Comirnaty BNT162b2. This includes for those who have
immunocompromised conditions and healthy women.
I have identified 2 memoranda in scope of this part of your request. These are reviews
presented to the COVID-19 Vaccine Independent Safety Monitoring Board (CV-ISMB) regarding
the safety of Comirnaty in pregnancy. These are itemised in Appendix 1 of this letter and copies
of the documents are enclosed. Where information is withheld, this is noted in the document
itself. I have considered the countervailing public interest in release in making this decision and
consider that it does not outweigh the need to withhold at this time.
On 3 November 2021 the Memo 'Temporary Medical Exemptions for the COVID-19
Vaccine: COVID-19 Vaccine Technical Advisory Group (CV TAG) recommendations'
stated 'Pregnancy is not a valid reason for exemption in the absence of any of the criteria
listed in the above table. Pregnancy is associated with higher risk from COVID-19
compared to the general population and therefore this group are a priority for vaccination.'
Q(2) As it is well known that doctors are extremely precautious and often refrain from
prescribing drugs without a long safety profile, please can you provide: (a) The latest reviews/reports/advice justifying and specifically applied as rationale for the
above statement that pregnancy is higher risk, as at November 2021.
In February 2022, the CV TAG recommended that 'A booster dose of the COVID-19
vaccine should be given from 3 months after the primary course to all eligible people aged
18 years and over, including immunocompromised individuals and pregnant persons.’
 (b) The scientific literature provided to doctors between October 2021- February 2022
(b) The scientific literature provided to doctors between October 2021- February 2022
justifying this recommendation.
Please refer to the following COVID-19 Vaccine Technical Advisory Group (CV TAG) memos for
all reports and data used to inform the decision available on the Manatū Hauora website here:
www.health.govt.nz/about-ministry/leadership-ministry/expert-groups/covid-19-vaccine-
technical-advisory-group-cv-tag.
These are provided as the relevant CV TAG memos up to and including, November 2021:
•
www.health.govt.nz/system/files/documents/pages/20210527_-
_cv_tag_recommendations_for_pfizer_covid-19_vaccine_in_pregnancy.pdf.
•
www.health.govt.nz/system/files/documents/pages/20211103_-_cv_tag_-
_medical_exemption.pdf.
These are provided as the relevant CV TAG memos up to and including, February 2022.
•
www.health.govt.nz/system/files/documents/pages/20211217_-_cv-tag_-
_booster_vaccinations_update.pdf.
• Temporary Medical Exemptions for the COVID-19 Vaccine: COVID-19 Vaccine
Technical Advisory Group (CV TAG) recommendations:
www.health.govt.nz/system/files/documents/pages/20220125_-_cv_tag_-
_booster_vaccinations_for_pregnant_people_and_immunocompromised.pdf.
•
www.health.govt.nz/system/files/documents/pages/20220201_-
_cv_tag_recommendations_on_booster_interval.pdf.
Q(3) Please provide the CV-TAG reviews/reports/advice which considered the risk benefit
of booster doses of Comirnaty BNT162b2 to pregnant women, including for those with
immunocompromised conditions and healthy women.
Please see the following CV TAG memos for all reports and data used to inform the decision:
•
www.health.govt.nz/system/files/documents/pages/20220201_-
_cv_tag_recommendations_on_booster_interval.pdf
• Update to recommendations on COVID-19 booster vaccinations for pregnant people and
immunocompromised: COVID-19 Vaccine Technical Advisory Group (CV TAG)
recommendations:
www.health.govt.nz/system/files/documents/pages/20220125_-
_cv_tag_-_booster_vaccinations_for_pregnant_people_and_immunocompromised.pdf
I trust this information fulfils your request. Under section 28(3) of the Act, you have the right to
ask the Ombudsman to review any decisions made under this request. The Ombudsman may
be contacted by email at:
[email address] or by calling 0800 802 602.
Please note that this response, with your personal details removed, may be published on the
Manatū Hauora website at:
www.health.govt.nz/about-ministry/information-releases/responses-
official-information-act-requests.
Nāku noa, nā
Jan Torres
Manager OIA Services
Government and Executive Services
Page 2 of 3
Appendix 1: List of documents for release
#
Date
Document details
Decision on release
1
23 March 2022
Memo: The Safety of COVID-19
Some information withheld
vaccination during pregnancy
under the following sections of
28963
the Act:
• 9(2)(g)(ii) to maintain
the effective conduct of
public affairs through
the protection of such
Ministers, members of
organisations, officers,
and employees from
improper pressure or
harassment
• 9(2)(a) of the Act, to
protect the privacy of
natural persons.
• section 9(2)(ba)(i) to
protect information that
is subject to an
obligation of
confidence and making
it available would likely
prejudice the supply of
similar information, or
information from the
same source.
2
22 October 2022
Memo: Pregnancy-related AEFI
Some information withheld
reports in New Zealand following under the following sections of
administration of
the Act:
Comirnaty
• 9(2)(g)(ii); and
• 9(2)(a),
Page 3 of 3

Document 1
Memo
Date:
23 March 2022
To:
s 9(2)(g)(ii)
, Manager, Clinical Risk Management, Medsafe
From:
s 9(2)(g)(ii)
, Clinical Risk Management
Subject:
The safety of COVID-19 vaccination during pregnancy
Incident ID:
28963
Lotus Notes Location: Immunological Products &
Vaccines – CV-ISMB
1982
For your:
Action: [√]
Decision: [√] Information: [√]
DESCRIPTION
ACT
This memo summarises the available information on safety and pregnancy outcomes when COVID-19
vaccines are administered during pregnancy. This memo covers new information that has become
available since the last memo dated 22 October 2021 (Annex 1). This information largely relates to
mRNA vaccines, including Comirnaty, which is the recommended vaccine for use during pregnancy in
New Zealand [1].
NATURE OF THE SAFETY CONCERN
Vaccination during pregnancy
Vaccine-preventable diseases can be associated with significant morbidity and mortality in pregnant
people, fetuses, and neonates. In some cases, immune system changes during pregnancy can increase
the susceptibility of the pregnant person and fetus to certain infectious diseases and increase the risk
of serious outcomes. Vaccination can provide direct protection of pregnant women, and can also
protect the fetus and infant through placental transfer of antibodies during pregnancy. COVID-19
INFORMATION
vaccination is known to be effective in the protection of pregnant women from COVID-19 disease.
Placental transfer of antibodies against SARS-CoV-2 has also been demonstrated [1-3].
RELEASED UNDER THE
There are no safety concerns surrounding administration of non-live vaccines during pregnancy.
Caution around administration of live attenuated vaccines such as the measles, mumps and rubella
(MMR) vaccine is based on the theoretical risk of placental transfer of attenuated virus and
subsequent infection of the foetus. However, evidence of foetal harm after vaccination has not been
identified. A review of the evidence around safety of vaccination during pregnancy by the Global
Advisory Committee on Vaccine Safety found no safety concerns with influenza, tetanus toxoid,
OFFICIAL
meningococcal, MMR, poliovirus or yellow fever vaccines [1, 2]. There is international consensus that
evidence indicates there are no pregnancy safety concerns with COVID-19 vaccines (see section on
recommendations from local and international bodies.
Page 1 of 23
Document 1
Risks of COVID-19 disease during pregnancy
The New Zealand immunisation handbook states:
‘Although pregnant women are not at increased risk of SARS-CoV-2 infection, they are at
increased risk of severe disease and death compared with age-matched non-pregnant women.
While the absolute risk of severe outcomes among pregnant women is low compared with
absolute risk due to advanced age, the risk of hospital admissions is three times higher and the
rate of ICU care for COVID‑19 has been found to be five times higher (relative risk 5.04; 95% CI
3.13–8.10) for pregnant women than for non-pregnant women. Obesity, hypertension, asthma,
autoimmune disease, diabetes and older age are also associated with severe COVID‑19 in
pregnant women.
Infants of mothers with COVID‑19 are at increased risk of preterm birth, particularly due to early
delivery, and neonatal ICU admission.[49, 52] Early studies do not suggest intrauterine
transmission, but transmission during birth has been shown in around 3 percent of neonates. Most
neonatal infections are asymptomatic or mild, but around 12 percent experience severe disease
with dyspnoea and fever as the most commonly reported signs.’
1982
Spontaneous abortion and stillbirth
THE
Spontaneous abortion or miscarriage is a non-viable pregnancy up to 20 weeks gestation. Most
commonly, this occurs during the first trimester, which is referred to as early pregnancy loss. Second
ACT
trimester pregnancy loss occurs after 13 and before 20 weeks gestation and stil -birth refers to
pregnancy loss at 20 weeks gestation or later [4].
The true incidence of early pregnancy loss is difficult to ascertain as many losses occur before the
pregnancy is clinical y recognised. The incidence of spontaneous abortion is thought to be around
UNDER
20% of clinical y recognised pregnancies, but has been estimated to be as high as 31% of al
pregnancies based on logistic regression.
There are more than 2,000 hospitalisations in New Zealand each year for spontaneous abortions. Most
people who experience a miscarriage do not require an inpatient stay in hospital, so this is a
significant undercount of the true number of people experiencing spontaneous abortion. In addition,
many people may miscarry without knowing they were pregnant. For these reasons, the total number
of miscarriages each year in New Zealand cannot be identified.
The risk of pregnancy loss changes with age. One study found rates of early pregnancy loss of 17
INFORMATION
percent (<20 years), 11 percent (20 to 24 years), 10 percent (25-29 years), 11 percent (30 to 34 years),
17 percent (35 to 39 years), and 33 percent (40 to 44 years). Other risk factors for pregnancy loss
RELEASED
include prior pregnancy loss, diabetes, obesity, thyroid disease, stress, use of certain medicines and
substance use. Some infections have been associated with increased risk of spontaneous abortion,
although the mechanism for this is unclear [4, 5].
Chromosomal abnormalities, maternal anatomic abnormalities and significant trauma may also cause
pregnancy loss. There can be multiple factors involved in second trimester pregnancy loss and often
no cause is identified [4].
OFFICIAL
The cause of a stil birth is often unknown. Congenital abnormalities, fetal growth restriction, infection,
genetic abnormalities, hydrops fetalis, fetal arrhythmia, abruptio placentae, umbilical cord
abnormalities, placental abnormalities and fetomaternal haemorrhage are known causes of stil birth
[6].
Page 2 of 23
Document 1
Sociodemographic risk factors for stillbirth include younger or older maternal age, nulliparity, parity
>3 and severe deprivation. Previous stil birth or adverse pregnancy outcome, diabetes, hypertension,
substance abuse and obesity are also risk factors [6].
The rates of stil birth in New Zealand vary from year to year and by demographic. There were 414
stillbirths in 2018, which equates to an overal rate of 7.0 per 1000 total births. The number of fetal
and infant deaths in New Zealand is smal and causes rates to fluctuate markedly from year to year. As
the rates in figure 1 are derived from small numbers, they should be interpreted with caution.
Figure 1: Rates of stillbirth, 1996-2018
9
8.5
8
7.5
7
1982
6.5
6
THE
5.5
5
ACT
1996
1997
1998
1999
2000
2001
2002
2003
2004
2005
2006
2007
2008
2009
2010
2011
2012
2013
2014
2015
2016
2017
2018
Source: New Zealand Mortality Collection via the Fetal and Infant Deaths Web Tool (accessed 1 March
2022). Note: Fetal death rates are expressed as per 1000 total births. Fetal deaths presented in this
UNDER
publication only include those meeting the definition of a stil birth (weighing ≥400 g birthweight or
who were ≥20 weeks gestation at birth). This includes deaths resulting from terminations of
pregnancy.
PRODUCTS
Product name
Sponsor
TT50
Comirnaty*
Pfizer New Zealand Limited
10853
*Comirnaty is the only vaccine currently recommended for use during pregnancy in New Zealand.
INFORMATION
INDICATIONS
RELEASED
Comirnaty is currently the only vaccine recommended for use during pregnancy in New Zealand. The
adult formulation of Comirnaty has provisional consent for active immunisation to prevent
coronavirus disease 2019 (COVID-19) caused by SARS-CoV2, in individuals 12 years of age and older.
The use of this vaccine should be in accordance with official recommendations.
INFORMATION IN LOCAL AND INTERNATIONAL PRODUCT INFORMATION
OFFICIAL
Section 4.6 of the New Zealand data sheet includes the fol owing information relating to use in
pregnancy:
‘There is limited experience with use of COMIRNATY in pregnant women. Animal studies do not
indicate direct or indirect harmful effects with respect to pregnancy, embryo/fetal development,
parturition or post-natal development (see Fertility). Administration of COMIRNATY in pregnancy
should only be considered when the potential benefits outweigh any potential risks for the mother
and fetus.’
Page 3 of 23
Document 1
The United Kingdom summary of product characteristics and Australian product information are
identical to the New Zealand data sheet.
The Canadian product monograph states:
‘The safety and efficacy of COMIRNATY in pregnant women have not yet been established. Animal
studies do not indicate direct or indirect harmful effects with respect to pregnancy, embryo/ fetal
development, parturition, or post-natal development.’
The United States prescribing information states:
’There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to
COMIRNATY during pregnancy. Women who are vaccinated with COMIRNATY during pregnancy
are encouraged to enrol in the registry by visiting https://mothertobaby.org/ongoing-
study/covid19-vaccines/.
Risk Summary
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the US general
1982
population, the estimated background risk of major birth defects and miscarriage in clinically
recognized pregnancies is 2% to 4% and 15% to 20%, respectively. Available data on COMIRNATY
THE
administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy.
A developmental toxicity study has been performed in female rats administered the equivalent of a
ACT
single human dose of COMIRNATY on 4 occasions; twice prior to mating and twice during
gestation. These studies revealed no evidence of harm to the fetus due to the vaccine.
Animal Data
In a developmental toxicity study, 0.06 mL of a vaccine formulation containing the same quantity
UNDER
of nucleoside-modified messenger ribonucleic acid (mRNA) (30 mcg) and other ingredients
included in a single human dose of COMIRNATY was administered to female rats by the
intramuscular route on 4 occasions: 21 and 14 days prior to mating, and on gestation days 9 and
20. No vaccine-related adverse effects on female fertility, fetal development, or postnatal
development were reported in the study.’
SOURCE OF SAFETY CONCERN
Review of safety data regarding vaccination during pregnancy is part of routine pharmacovigilance
INFORMATION
activities. There have been reports of AEFIs after vaccination during pregnancy (see section on
spontaneous reporting data in New Zealand) and there is considerable public interest in the safety
RELEASED
profile during pregnancy.
REVIEW OF THE AVAILABLE INFORMATION
Spontaneous reporting data in New Zealand
General reporting patterns
OFFICIAL
As of 22 March 2022, there were a total of 60,378 cases in the database of which 569 had the
pregnancy checkbox selected by the reporter. When restricted to females aged 16-49 years, 462
report remain (67 serious). Al reports were for Comirnaty, aside from one case for Vaxzevria. The total
number of people that have been vaccinated during pregnancy is unknown, as this information is not
recorded at the point of vaccination.
The overal spontaneous reporting trends for cases marked as pregnant are similar to those for the
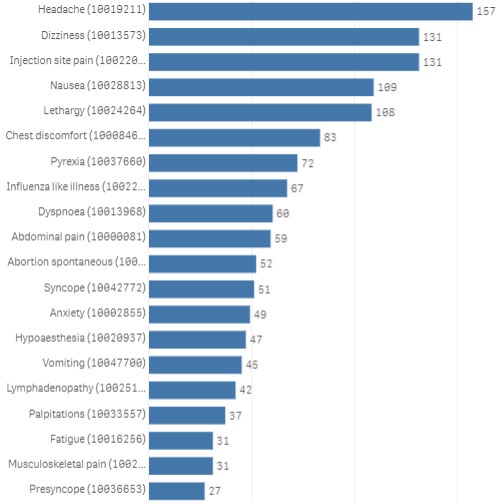
general population. Figure 2 shows the top reported terms for cases marked as pregnant in females
Page 4 of 23
Document 1
aged 16-49 years, which are also frequently reported in the overal population, with the exception of
spontaneous abortion.
Table 1 compares the proportion of these cases reporting each term and compares this to the overal
population. The proportion of cases reporting each term is similar between the pregnant cases and
general population. Reporting rates were unable to be calculated as the total number of doses
administered to pregnant people is unknown.
When compared to overal reports, it appears that a higher proportion of the pregnant cases reported
vomiting (9.7% vs 5.1%) and abdominal pain (12.8% vs 7.7%). Conversely, a lower proportion of
pregnant cases appeared to report lymphadenopathy (9.1% vs 11.9%). These differences are smal and
highly uncertain due to the limitations of passive reporting. However, the terms reported and general
patterns are consistent with international experience and the literature, and do not highlight any
safety concerns.
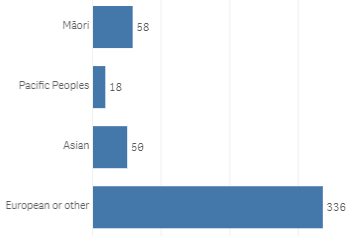
Figures 3 and 4 show the age and ethnicity of pregnant cases.
Figure 2: Most frequently reported adverse report terms for cases marked as pregnant in females aged
1982
16-49 years
THE
ACT
UNDER
INFORMATION
RELEASED
OFFICIAL
Source: COVID-19 Adverse Events Fol owing Immunisation Qlik app, updated 22 March 2022
(accessed 22 March 2022).
Page 5 of 23
Document 1
Table 1: Proportion of cases reporting the most frequent AEFI terms for cases marked as pregnant in
females aged 16-49 years, compared with the overall population
AEFI term
Percentage of pregnant
Percentage of al cases
cases reporting AEFI
reporting AEFI
Headache
34.0%
31.2%
Dizziness
28.4%
29.3%
Injection site pain
28.4%
25.6%
Nausea
23.6%
21.1%
Lethargy
23.4%
24.4%
Chest discomfort
18.0%
20.3%
Pyrexia
15.6%
14.4%
1982
Influenza like illness
14.5%
13.7%
Dyspnoea
13.0%
11.7%
THE
Abdominal pain
12.8%
7.7%
ACT
Abortion spontaneous*
11.3%
n/a
Syncope
11.0%
9.4%
Anxiety
10.6%
8.5%
Hypoaesthesia
10.2%
9.9%
UNDER
Vomiting
9.7%
5.1%
Lymphadenopathy
9.1%
11.9%
Palpitations
8.0%
7.8%
Fatigue
6.7%
4.7%
Musculoskeletal pain
6.7%
6.0%
INFORMATION
Presyncope
5.8%
4.9%
Source: COVID-19 Adverse Events Fol owing Immunisation Qlik app, updated 22 March 2022
RELEASED
(accessed 22 March 2022).
*Note that there are additional cases of spontaneous abortion that are not marked as pregnant.
OFFICIAL
Page 6 of 23

Document 1
Figure 3: Age groups of pregnant cases
1982
THE
Figure 4: Ethnicity of pregnant cases
ACT
UNDER
INFORMATION
Serious cases
RELEASED
The terms reported for the 67 serious cases were spontaneous abortion (41), chest discomfort (12),
abdominal pain (11), headache (11), dyspnoea (10), anxiety (9), dizziness (9), lethargy (8), injection site
pain ( 7), nausea (7), pyrexia (7), syncope (7), vaginal haemorrhage (7), hypoaesthesia (6), presyncope
(5) influenza like illness (4) palpitations (4), fatigue (3), insomnia (3), menstrual disorder (3), premature
labour (3), pulmonary embolism (3), rash (3), tachycardia (3), vomiting (3), wheezing (3), abortion
missed (2), disturbance in attention (2), exposure during pregnancy (2), oedema peripheral (2),
OFFICIAL
paraesthesia (2), rash erythematous (2) rash pruritic ( 2), stillbirth (2), swelling (2), vision blurred (2),
weight decreased (2), abortion (1), ageusia (1), alopecia areata (1), arthralgia (1), bronchospasm (1),
congenital anomaly (1), decreased appetite (1), depressed level of consciousness (1), erythema
multiforme (1), face oedema (1), feeling of body temperature change ( 1), haemorrhage (1),
hypokinesia (1), injection site paraesthesia (1), injection site pruritus (1), lymphadenopathy (1),
musculoskeletal pain (1), myalgia (1), nephrotic syndrome (1), periorbital oedema (1), photophobia (1),
Page 7 of 23


Document 1
pruritus (1), restlessness (1), seizure (1), sleep disorder (1), suicide attempt (1), superficial vein
thrombosis (1), throat tightness (1), tinnitus (1), urticaria (1), vertigo (1).
It should be noted that these terms include those selected by the reporter and may not be medically
confirmed.
Cases reporting pregnancy loss
As of 22 March 2022, there were 66 reports coded with the terms spontaneous abortion, abortion or
missed abortion and two cases coded with stillbirth. There are also three reports of fetal hypokinesia
and one report of congenital abnormality, which was included in the October 2021 memo. The details
of the cases are provided in Annex 2.
Of the 66 cases reporting spontaneous abortion, abortion or missed abortion, 49 occurred in the first
trimester, 5 occurred in the second trimester and 12 occurred at unknown gestation or stated early
pregnancy. The two cases of stil birth occurred at 29 and 39 weeks. Figures 5 and 6 show the age and
ethnicity of the reported cases of pregnancy loss.
1982
Figure 5: Age of cases reporting pregnancy loss
THE
ACT
UNDER
Figure 6: Ethnicity of cases reporting pregnancy loss
INFORMATION
RELEASED
OFFICIAL
Page 8 of 23
Document 1
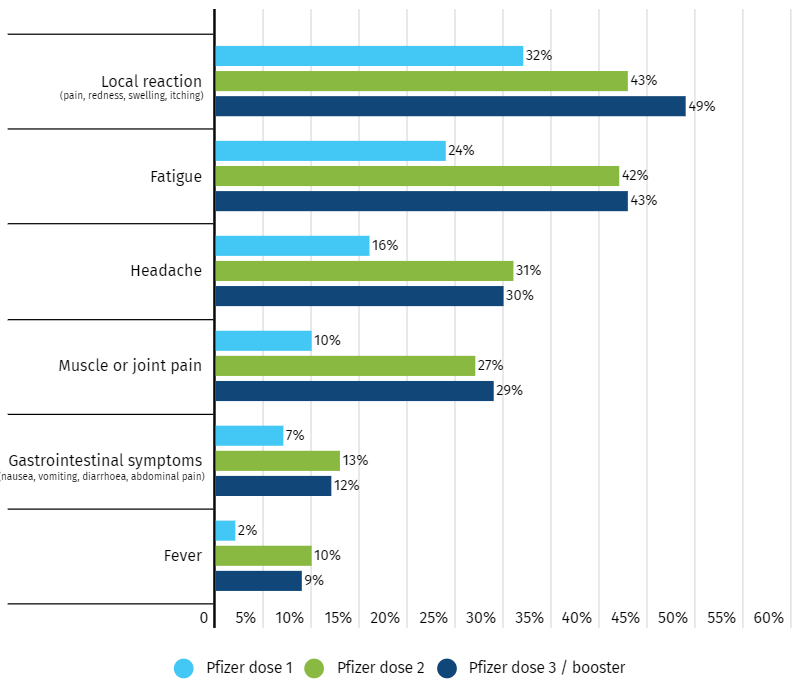
Solicited reporting in Australia
Australia has published results from AusVaxSafety, an active surveil ance programme that includes
COVID-19 vaccines. Information on adverse events is col ected with surveys sent by text message or
email at day three, day eight and six weeks after vaccination. Data from surveys completed by
pregnant people at day 3 after vaccination with Comirnaty is summarised on the AusVaxSafety
website.
As of 21 March 2022, there were 11,182 surveys completed after dose one, 12,118 surveys after dose
two and 6,480 surveys after dose three. After doses one, two and three, 37%, 52% and 55% of
respondents, respectively, reported at least one adverse event. Figure 7 shows the most frequently
reported adverse events. Up to 2% of respondents reported seeking medical attention and up to 23%
reported disruption of routine activities after each dose. People who presented to GPs and emergency
departments had similar adverse events to those who didn’t. Most people who reported disruption of
routine activities had lethargy, headache and joint pain.
Figure 7: Frequently reported adverse events reported by pregnant participants at day three following
vaccination with Comirnaty. AusVaxSafety, 2022 [7]
1982
THE
ACT
UNDER
INFORMATION
RELEASED
OFFICIAL
Page 9 of 23
Document 1
Recommendations from local and international bodies
The New Zealand Ministry of Health, COVID-19 Vaccine Independent Safety Monitoring Board, Royal
Australian and New Zealand Col ege of Obstetricians and Gynaecologists, Royal College of
Obstetricians and Gynaecologists (UK), Joint Committee on Vaccination and Immunisation (UK),
Canadian Ministry of Health, Centres for Disease Control and Prevention and European Medicines
Agency have published communications in support of the safety of routine COVID-19 vaccination in
pregnant people [8-13].
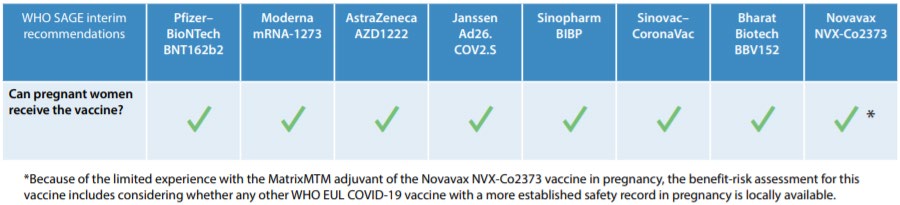
The WHO Strategic Advisory Group of Experts on Immunization (SAGE) recommends that pregnant
people who are not already vaccinated against COVID-19 should have access to COVID-19
vaccination, because of the increased risk of severe il ness and preterm birth. The WHO considers that
the benefits of vaccination during pregnancy outweigh potential risks whenever there is ongoing or
anticipated community transmission of the virus [3].
Table 2: WHO SAGE interim recommendations on vaccination during pregnancy [3]
1982
THE
ACT
Literature
UNDER
In the previous memo on this topic, dated 22 October 2021, the available literature on COVID-19
vaccine safety and pregnancy outcomes after vaccination was summarised.
Shimabukuro et al examined data from the CDC v-safe COVID-19 Vaccine Pregnancy Registry and
found the proportions of pregnancies with preterm birth or being smal for gestational age at birth
were consistent with background rates [14]. Blakeway et al found no difference between vaccinated
and unvaccinated people for a range of adverse pregnancy outcomes [15]. Theiler et al and Trostle et
al found no increased risk of maternal, pregnancy or delivery complications after vaccination [16, 17].
Kharbanda et al found that among women with spontaneous abortions, the odds of COVID-19 vaccine
INFORMATION
exposure were not increased in the prior 28 days compared with women with ongoing pregnancies
[18]. Zauche et al found that the risk of spontaneous abortion after mRNA Covid-19 is consistent with
RELEASED
the expected background risk [19].
Bookstein et al and Kachikis et al found that the short-term safety profile following vaccination is
comparable to non-pregnant people [20, 21].
Relevant literature identified since the previous memo dated 22 October 2021 is summarised below.
Most of the literature relating to pregnancy outcomes after vaccination includes participants
predominantly vaccinated during the second or third trimester. Further accrual of fol ow-up time is
OFFICIAL
needed to observe large numbers of pregnancy outcomes in people vaccinated during the first
trimester. However, the first trimester data that exist do not raise any safety concerns.
New studies have also been published on fertility and the general safety profile in pregnancy.
Page 10 of 23
Document 1
Literature on pregnancy outcomes
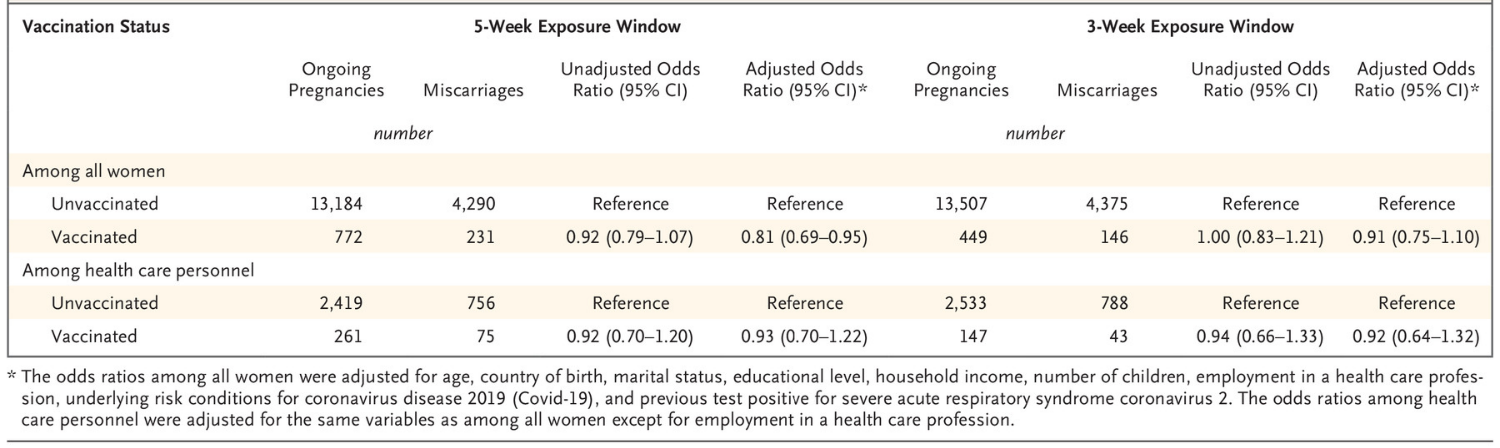
Magnus et al, 2021. COVID-19 vaccination during pregnancy and first-trimester miscarriage [22]
This case control study, summarised in a letter to the editor, estimated the odds of COVID-19
vaccination in people who had first trimester miscarriage (cases) compared with people with a primary
care confirmation of ongoing pregnancy in the first trimester (controls).
The data was derived from Norwegian registries and al registrations of first trimester miscarriages or
ongoing first trimester pregnancies between 15 February 2021 and 15 August 2021 were identified. At
the time of the study, vaccination during pregnancy was not recommended in Norway during the first
trimester except in people underlying health conditions and the proportion of vaccinated people in
the study was smal (around 5%).
The authors estimated odds ratios for COVID-19 vaccination within 5-week and 3-week windows
before a miscarriage or confirmed ongoing pregnancy. Adjustments were made for age, country of
birth, marital status, educational level, household income, number of children, employment in a health
care profession, underlying risk conditions for COVID-19, previous positive test for SARS-CoV-2, and
1982
calendar month.
THE
There were 13,956 ongoing pregnancies (5.5% vaccinated) and 4,521 miscarriages (5.1% vaccinated)
identified. For people who miscarried, the adjusted odds ratios were 0.91 (95% CI: 0.75 to 1.10) for
vaccination within the prior three weeks and 0.81 (95% CI: 0.69 to 0.95) for vaccination within the prior
ACT
five weeks (Table 5). Separate analyses were conducted with similar results for different vaccines,
health care workers, and confirmed pregnancies with at least eight weeks of fol ow up to exclude
subsequent pregnancy loss. The study did not find an association between COVID-19 vaccination and
early pregnancy loss.
UNDER
Table 3: Odds ratios for COVID-19 vaccination in a 5-week or 3-week window before miscarriage or
confirmation of an ongoing pregnancy. Magnus et al, 2021 [22]
INFORMATION
RELEASED
One limitation of the study was the inability to match for gestational age at registration, although the
authors considered that most recognised miscarriages occur between weeks six and ten of pregnancy,
which is similar to the period in which most pregnancies are confirmed in primary care. Other
limitations were that many people in Norway do not have a primary care appointment to confirm
OFFICIAL
pregnancy and some miscarriages are not clinically recognised.
Wainstock et al, 2021. Prenatal maternal COVID-19 vaccination and pregnancy outcomes [23]
This single-centre retrospective cohort study compared the odds of various pregnancy and neonatal
outcomes between people vaccinated with Comirnaty and unvaccinated people. The study included all
women who delivered live babies between January and June 2021 at Soroka University Medical
Center, Israel. People with previous SARS-CoV-2 infection, multiple gestations or unknown vaccination
Page 11 of 23
Document 1
status or incomplete pregnancy fol ow up information were excluded. Al vaccinations occurred during
the third trimester.
The final study population included 913 vaccinated people and 3,486 unvaccinated people (before or
during pregnancy). Vaccinated women were older, more likely to receive fertility treatment, less likely
to receive insufficient prenatal care and had higher socioeconomic status.
A multivariate analysis was conducted that adjusted for maternal age, fertility treatments and
socioeconomic score (table 6). No adverse associations were found between third trimester
vaccination and any of the pregnancy or neonatal complications.
Table 4: Multivariable models for the association between vaccination and pregnancy, delivery and
newborn characteristics and complications. Wainstock et al , 2021 [23]
Outcomes
Adjusted Odds ratio* (vaccinated
vs. unvaccinated); 95% CI
Pregnancy complications diagnosed in late pregnancy
1982
Pregnancy related hypertensive disorders
1.13; 0.78–1.62
THE
Oligohydramnios
0.84; 0.52–1.40
Polyhydramnios
0.77; 0.29–2.03
ACT
Pathological presentation
0.96; 0.63–1.48
Meconium stained amniotic fluid
0.52; 0.32–0.83
Delivery and post-partum characteristics
UNDER
Gestational age at delivery
β = −0.07; (−0.26–0.11)
Non reassuring fetal monitoring
0.70; 0.48–1.01
Caesarean delivery
0.93; 0.75–1.16
Vacuum delivery
0.99; 0.63–1.57
Postpartum haemorrhage
1.46; 0.63–3.38
INFORMATION
Maternal postpartum fever
0.73; 0.15–3.51
Newborn characteristics
RELEASED
Birthweight, gr. (mean ± SD)
β = −9.14; (−55–37.5)
Small for gestational age
0.79; 0.48–1.31
Newborn postpartum fever
1.45; 0.26–8.11
Newborn respiratory complications
0.88; 0.44–1.79
OFFICIAL
*All models adjusted for maternal age, fertility treatments and socioeconomic score
The authors noted that the study was insufficiently powered to detect differences between exposure
to one dose versus two doses. Women were categorised as exposed regardless of the time between
vaccination and birth, which ranged from one to 21 weeks. The numbers of pregnancies associated
with each outcome were smal .
Page 12 of 23
Document 1
Rottenstreich et al, 2021. Covid-19 vaccination during the third trimester of pregnancy: rate of
vaccination and maternal and neonatal outcomes, a multicentre retrospective cohort study [24]
This retrospective cohort study aimed to compare composite adverse maternal outcomes and
composite adverse neonatal outcomes between people vaccinated with Comirnaty in the third
trimester and unvaccinated pregnant people. The study was conducted at two medical centres that
account for 16% of deliveries in Israel between 19 January 2021 and 27 April 2021. People with current
or previous COVID-19 disease were excluded.
The composite adverse maternal outcome included chorioamnionitis, postpartum haemorrhage,
endometritis, blood transfusion, a caesarean delivery, ICU admission and a maternal hospital length of
stay of longer than five days for vaginal delivery and longer than seven days for caesarean delivery.
Some of these outcomes were also assessed individual y. Secondary outcome analyses were only
performed for people who received two doses of the vaccine.
The composite adverse neonatal outcome included intrauterine fetal death (IUFD), Apgar score of ≤7
at 1 minute, Apgar score of ≤7 at 5 minutes, admission to neonatal intensive care unit, neonatal
asphyxia, intracranial haemorrhage, meconium aspiration syndrome, hyperbilirubinaemia, neonatal
1982
seizures, neonatal hypoglycaemia, neonatal sepsis and use of mechanical ventilation. These outcomes
were also assessed individually.
THE
There were 1,775 deliveries included in the study, of which 712 were in vaccinated people and 1,063
ACT
were in unvaccinated people. Those who had received two doses of the vaccine were older, and more
likely to have had previous miscarriage, caesarean delivery or fertility treatment.
The proportion of deliveries affected by the composite adverse maternal outcome was not
significantly different between vaccinated and unvaccinated people (24.2% vs 23.6%, p=0.79). In the
multivariate analysis, the adjusted odds ratio for the composite maternal outcome was 0.8 (95% CI
UNDER
0.61–1.03).
The proportion of deliveries affected by the composite adverse neonatal outcome was significantly
lower in the vaccinated group compared with the unvaccinated group (7.9% vs 11.4%, p=0.02). The
adjusted odds ratio for the composite neonatal outcome was 0.5 (95% CI 0.36–0.74).
The study did not find an association between COVID-19 vaccination during the third trimester and
poorer maternal or neonatal outcomes. The authors note that people with asymptomatic previous or
current SARS-CoV-2 infection may have been inadvertently included in the study. Information on the
INFORMATION
interval between vaccination and delivery was not available. The numbers of deliveries with rarer
adverse outcomes were too smal to detect any potential differences between the vaccinated and
RELEASED
unvaccinated group.
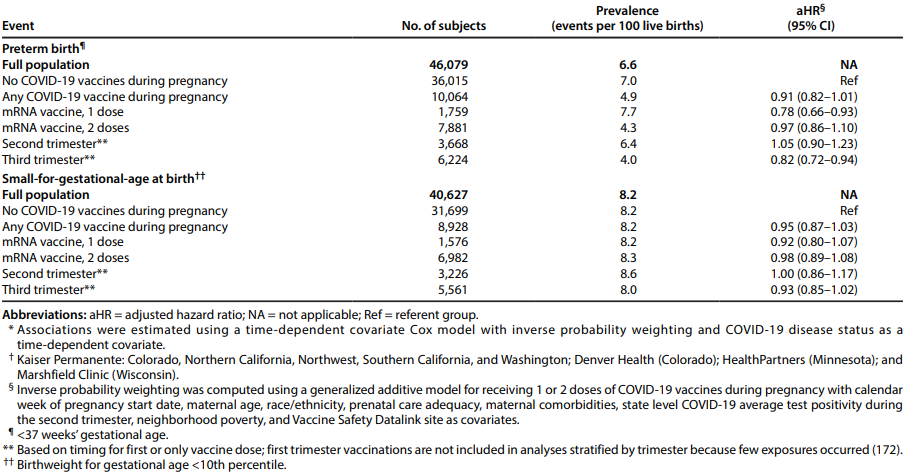
Lipkind et al, 2022. Receipt of COVID-19 vaccine during pregnancy and preterm or small-for-
gestational-age at birth — eight integrated health care organizations, United States, December 15,
2020-July 22, 2021 [25]
This report is an early release from the Centres for Disease Control and Prevention via the Morbidity
and Mortality Weekly Report. This retrospective cohort study evaluates if there is an association
OFFICIAL
between COVID-19 vaccination and preterm birth or smal -for-gestational-age at birth, accounting for
time-dependent vaccine exposures and propensity to be vaccinated. Pregnancies with estimated start
or last menstrual period between 17 May 2020 and 24 October 2020 were eligible for inclusion and
multiple gestation pregnancies were excluded. The data was obtained from the Vaccine Safety
Datalink, which col ects electronic health data from nine health care organisations representing three
percent of the United States population.
Page 13 of 23
Document 1
There were 46,079 pregnant people with live births and gestational age available, of whom 10,064
received at least one dose of a COVID-19 vaccine. Nearly al (98.3%) of these people were vaccinated
during the second or third trimester. There was no association between vaccination and preterm birth
(adjusted hazard ratio [aHR] 0.91; 95% CI 0.82–1.01). There was also no association between
vaccination and being smal for gestational age at birth (aHR 0.95; 95% CI 0.87–1.03). There was also
no increased risk when results were stratified by vaccine dose or trimester of vaccination (table 7).
Table 5: Preterm births, smal -for-gestational-age births, and adjusted hazard ratios* among women
receiving COVID-19 vaccine during pregnancy compared with unvaccinated pregnant women — eight
U.S. health care organizations,† December 15, 2020–July 22, 2021. Lipkind et al, 2022 [25]
1982
THE
ACT
UNDER
The authors identified that some vaccinations may have been missed, causing possible bias towards
the null. There was also missing information on confounders such as previous SGA or preterm birth
and previous SARS-CoV-2 infection. The decreased risks of preterm birth with third trimester
vaccination and receipt of only one dose were thought to be due to residual immortal time bias.
INFORMATION
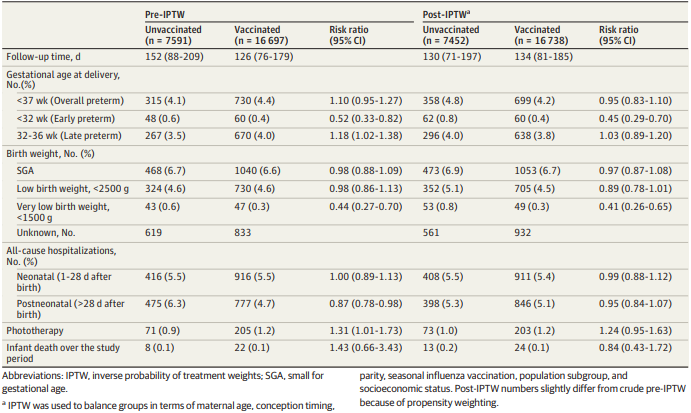
Goldshtein et al, 2022. Association of BNT162b2 COVID-19 vaccination during pregnancy with neonatal
and early infant outcomes [26]
RELEASED
This cohort study aimed to examine whether vaccination with Comirnaty during pregnancy is
associated with adverse neonatal and early infant outcomes. Data were extracted from a public health
fund database that represents 26.7% of the population of Israel. Records with missing maternal
linkage or important covariate data were excluded. The study included al singleton births between 1
March 2021 and 31 September 2021. The primary outcomes were smal birth weight for gestational
age (SGA) and preterm birth. Exploratory outcomes included inpatient hospitalisations, recorded
OFFICIAL
congenital anomalies, jaundice requiring phototherapy, and al -cause death over the study period.
After exclusions, 24,288 eligible newborns were identified, of whom 16,697 were born to mothers
vaccinated during pregnancy. The vaccinated group had older maternal age at birth, higher influenza
vaccine uptake, lower likelihood of belonging to an ethnic minority and greater likelihood of living in
more affluent areas.
The study found no association between vaccination and SGA (RR = 0.97; 95% CI, 0.87-1.08) or overall
preterm birth (RR = 0.95; 95% CI, 0.83-1.10) (table 8). An analysis of first trimester vaccination showed
Page 14 of 23
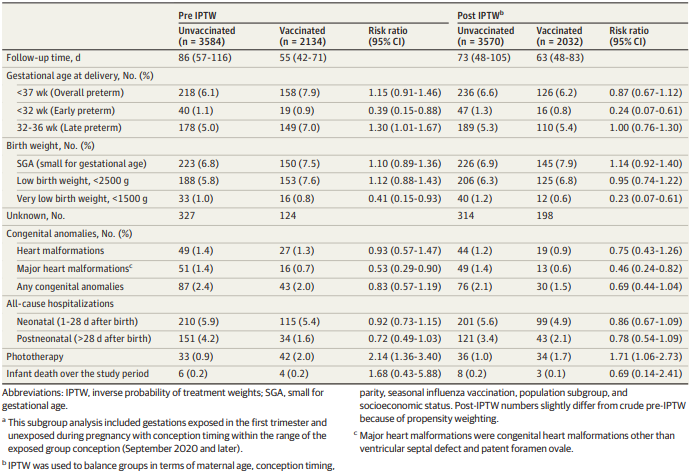
Document 1
similar results and also found no adverse association with congenital abnormalities (table 9). No
association was found for the exploratory outcomes of hospitalisations and infant death. An
association was found between vaccination and jaundice requiring phototherapy in the first trimester
analysis and in the sensitivity analysis where mothers with prior SARS-CoV-2 infection were excluded.
The authors attributed this to possible confounding from a higher rate of smoking in the vaccinated
group. The numbers of these rarer outcomes are too smal to draw definitive conclusions.
Table 6: Early neonatal and infant outcomes. Goldshtein et al, 2022 [26]
1982
THE
ACT
UNDER
Table 7: Neonatal and early infant outcomes for first trimester vaccination. Goldshtein et al, 2022 [26]
INFORMATION
RELEASED
OFFICIAL
Page 15 of 23
Document 1
This study is underpowered to detect potential differences in rarer outcomes fol owing first trimester
vaccination and accumulation of further fol ow-up time is needed. Another limitation is that the study
population is limited to newborns registered in the database and may not capture al cases of very
early infant mortality.
Literature on general safety profile in pregnant people
Sadarangani et al, 2022. Safety of COVID-19 vaccines in pregnancy: a Canadian National Vaccine Safety
(CANVAS) Network study (preprint) [27]
This pre-print study aimed to determine significant health events amongst pregnant females after
COVID-19 vaccination, compared with unvaccinated pregnant controls and vaccinated non-pregnant
individuals. Participants were actively recruited and asked to complete surveys via email on any AEFIs
during the seven days fol owing each vaccine dose, or in the prior seven days in the case of
unvaccinated participants. The study included females reporting pregnancy and non-pregnant females
from the age groups.
1982
The primary endpoint was ‘significant health event’, defined as a new or worsening health event that
caused absence from work or school, medical consultation or prevented normal activities. ‘Serious
THE
health event’ was a secondary endpoint, defined as any event resulting in emergency department visit
or hospitalisation. Al events were self-reported and not medically confirmed.
ACT
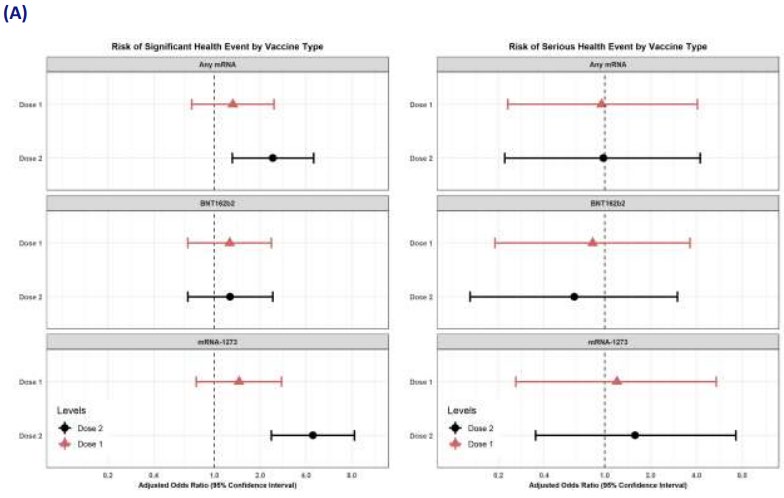
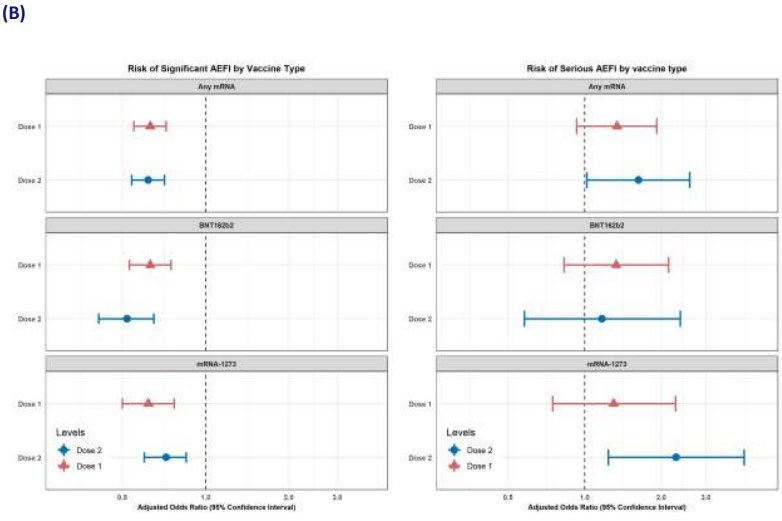
Of the mRNA-vaccinated pregnant individuals, 4.0% (226) and 7.3% (227) reported a significant health
event after dose one and dose two, respectively. The most frequently reported events were malaise,
myalgia, headache and respiratory tract infection. By comparison, 3.2% (11) pregnant unvaccinated
participants reported a significant health event. Serious health events were reported by 0.6%-0.9% of
pregnant participants, depending on vaccine type. Miscarriage/stil birth was reported at similar rates
UNDER
between unvaccinated and vaccinated participants after dose one (n=7 [2.1%] and n=83 [1.5%],
respectively).
In the multivariate analysis that adjusted for age group, prior COVID-19 infection and trimester (figure
8), there was an increased risk of a significant health event within seven days of dose two of any
mRNA vaccine (aOR: 2.4; 95% CI: 1.3-4.5) or dose two of Spikevax (aOR: 4.4, 95% CI: 2.4-8.3) for
pregnant vaccinated individuals, compared with pregnant unvaccinated controls. These associations
disappeared in the sensitivity analyses that were restricted to participants reporting good health
status and events requiring medical care. There was no association between vaccination and serious
INFORMATION
health events.
RELEASED
When comparing vaccinated pregnant and vaccinated non-pregnant people, significant AEFI rates
(excluding injection site reactions) were consistently lower among pregnant people across al mRNA
vaccine types and doses. Overal , 4.0% and 7.3% of pregnant people reported a significant AEFI after
dose one and dose two, respectively, compared with 6.3% and 11.3% for non-pregnant people.
In the multivariate analysis (figure 8), pregnancy was associated with a decreased risk of significant
health events for any mRNA vaccine or dose. There was no association between pregnancy status and
significant health events when the analysis was restricted to events requiring medical care. For the
OFFICIAL
secondary endpoint of serious events, dose two of Spikevax was associated with a higher risk in
pregnant participants compared with non-pregnant participants (aOR 2.3; 95% CI: 1.2-4.2). It should
be noted that this result is based on very smal numbers of serious events in pregnant people (11).
Limitations of this study include the potential for recall bias, lack of medical confirmation of serious
events and relatively smal sample size.
Page 16 of 23
Document 1
Figure 8: Multivariable logistic regression analyses comparing significant and serious health events
amongst (A) pregnant people, comparing vaccinated with unvaccinated individuals and (B) vaccinated
people, comparing pregnant with non-pregnant individuals. Sadarangani et al, 2022. [27]
1982
THE
ACT
UNDER
INFORMATION
RELEASED
OFFICIAL
Page 17 of 23
Document 1
Nakahara et al, 2022. Safety-related outcomes of novel mRNA COVID-19 vaccines in pregnancy [28]
This cohort study examined the safety profile of mRNA COVID-19 vaccination in 83 pregnant people
and 166 age-matched controls vaccinated between December 2020 and January 2021. The primary
outcome was frequency of any vaccine-related complaint and secondary outcomes included specific
complaints and positive COVID-19 test.
The frequency of complaints fol owing vaccination was not different between pregnant and non-
pregnant patients (18.1 vs. 16.9%, p = 0.201). Pregnant patients were more likely to report fever (4.8
vs. 0.6%, p = 0.044) and gastrointestinal symptoms (4.8 vs. 0%, p = 0.012).
Literature on fertility
Aharon et al, 2022. In vitro fertilization and early pregnancy outcomes after Coronavirus Disease 2019
(COVID-19) vaccination [29]
This retrospective cohort study examined whether COVID-19 vaccination was associated with
differences in fertilisation rate in people who underwent controlled ovarian hyperstimulation (COH) or
1982
single euploid frozen-thawed embryo transfer (FET) at a single academic centre. Secondary outcomes
for COH included eggs retrieved, mature oocytes retrieved, mature oocytes ratio, blastulation rate,
THE
and euploid rate. Secondary outcomes for FET included pregnancy rate, ongoing pregnancy rate,
biochemical pregnancy loss rate, and clinical pregnancy loss rate.
ACT
The exposed group consisted of patients who had received two doses of either Comirnaty or Spikevax
at least 14 days before starting medication for their procedure and the control group consisted of
unvaccinated patients. The first cycle for each patient between February and September 2021 was
included.
UNDER
The COH group included 222 fully vaccinated patients and 983 unvaccinated patients. The adjusted
analysis found no association between vaccination and fertilisation rate (β = 0.02 ± 0.02, P = 0.20) or
any of the secondary outcomes.
The FET group included 214 vaccinated patients and 733 unvaccinated patients. The adjusted analysis
found no association between vaccination and fertilisation rate (aOR = 0.79, 95% CI 0.54–1.16) or any
of the secondary outcomes.
One strength of this study is that it captures early implantation and early pregnancy losses that may
be unrecognised in other studies. These findings provide further reassurance that COVID-19
INFORMATION
vaccination is not associated with impaired fertility or early pregnancy losses. Limitations include
unknown SARS-CoV-2 infection status of the participants and smal number of vaccinated participants.
RELEASED
Fetal and birth outcomes were not assessed in this study.
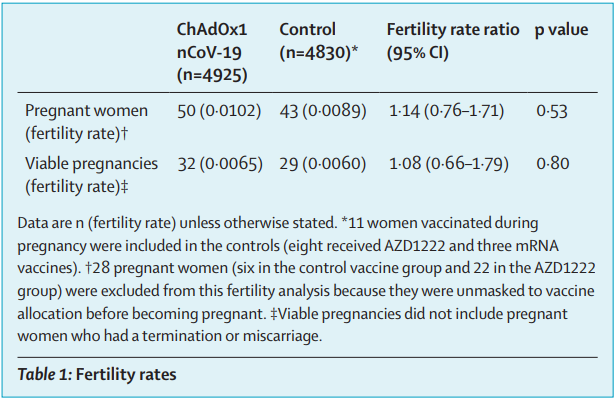
Hillson et al, 2021. Fertility rates and birth outcomes after ChAdOx1 nCoV-19 (AZD1222) vaccination
[30]
This correspondence published in the Lancet analyses the pregnancies that have occurred in four
ongoing clinical trials for Vaxzevria. Pregnant people were excluded from the trials, but any
pregnancies occurring after vaccination are fol owed up until three months after birth.
OFFICIAL
The fertility outcome analysis set included 93 pregnant women (50 vaccinated and 43 control). There
was no significant difference in fertility of vaccinated and unvaccinated participants as measured by
the total number of pregnancies or by viable pregnancies (table 11).
Page 18 of 23

Document 1
Table 8: Fertility rates. Hillson et al, 2021 [30]
The pregnancy outcome analysis set included 107 women (72 vaccinated and 35 control). Controls
1982
who were subsequently vaccinated were excluded from the analysis. There were no differences in the
pregnancy outcomes of miscarriage or termination, or preterm birth (table 12). Analyses that exclude
Brazilian data were conducted as pregnancy termination is il egal in Brazil. Most pregnancies were stil
THE
ongoing at the time of analysis. There were no stil births or neonatal deaths; however, this paper
defines miscarriage as pregnancy loss before 23 weeks gestation while the New Zealand definition is
ACT
before 20 weeks.
Table 9: Pregnancy outcomes. Hillson et al, 2021 [30]
UNDER
INFORMATION
RELEASED
Periodic Benefit Risk Evaluation Reports (PBRERs)
s 9(2)(ba)(i)
OFFICIAL
Page 19 of 23
Document 1
s 9(2)(ba)(i)
E
1982
XPERT ADVICE
This memo will be presented to the COVID-19 Vaccine Independent Safety Monitoring Board (CV-
THE
ISMB).
ACT
CONCLUSION AND PROPOSED ACTIONS
Pregnancy is associated with increased risk of severe COVID-19 disease and death. The risk of hospital
admissions has been found to be three times higher and the rate of ICU care five times higher than in
non-pregnant women. Obesity, hypertension, asthma, autoimmune disease, diabetes and older age
are also risk factors for severe COVID-19 disease during pregnancy. Infants of infected mothers have
UNDER
increased risks of preterm delivery and neonatal ICU admission [1].
As of 22 March 2022, of a total of 60,378 cases, there were 462 AEFI case reports in females aged 15-
49 years where pregnancy was indicated using the checkbox on the reporting form. Al reports were
for Comirnaty apart from one report for Vaxzevria. The reporting patterns were similar to that of the
general population. The most frequently reported events were those expected after vaccination, such
as headache, dizziness, injection site pain, nausea and lethargy.
As of 22 March 2022, there were 66 reports of spontaneous abortion, abortion or missed abortion and
INFORMATION
two reports of stil birth. Of the 66 cases reporting spontaneous abortion, abortion or missed abortion,
49 occurred in the first trimester, 5 occurred in the second trimester and 12 occurred at during early
RELEASED
pregnancy or unknown gestation. Spontaneous abortion is common, occurring in around 1 in 5
clinical y recognised pregnancies. The reports received do not highlight any safety concerns, although
they contain limited information.
Scientific literature supporting the safety of COVID-19 vaccination in pregnancy has accumulated
since the previous memo dated 22 October 2022. The studies published to date have not found an
increased risk of a range of maternal or neonatal adverse pregnancy outcomes, including preterm
birth, smal for gestational age at birth and spontaneous abortion [22-26]. No adverse effects on
OFFICIAL
fertility have been detected [29, 30].
There are no safety concerns in general with the use of non-live vaccines in pregnancy. The New
Zealand spontaneous reporting data and scientific literature overwhelmingly support the safety
COVID-19 vaccination in pregnancy. Routine pharmacovigilance activities should continue.
Page 20 of 23
Document 1
RECOMMENDATIONS
It is recommended that:
1.
Routine pharmacovigilance activities are continued
Yes/No
REFERENCES
1.
Ministry of Health. 2020.
Immunisation Handbook 2020 September 2020. URL:
https://www.health.govt.nz/our-work/immunisation-handbook-2020 (accessed 12 October
2021).
2.
Global Advisory Committee on Vaccine Safety. 2014.
Safety of immunization during pregnancy:
a review of the evidence July 2014. URL:
https://www.who.int/publications/i/item/safety-
immunization-pregnancy (accessed 12 October 2021).
3.
World Health Organization. 2022.
Questions and answers: COVID-19 vaccines and pregnancy 15
1982
February 2022. URL:
https://www.midwife.org.nz/wp-content/uploads/2021/09/WHO-QAs-
COVID-19-vaccines-pregnancy_en.pdf (accessed 22 March 2022).
THE
4.
UpToDate. 2021.
Pregnancy loss (miscarriage): Terminology, risk factors, and etiology 24
September 2021. URL:
https://www.uptodate.com/contents/pregnancy-loss-miscarriage-
ACT
terminology-risk-factors-and-etiology (accessed 21 October 2021).
5.
Ministry of Health. 2017.
Miscarriage and stillbirth 30 May 2017. URL:
https://www.health.govt.nz/your-health/pregnancy-and-kids/services-and-support-during-
pregnancy/miscarriage-and-stillbirth (accessed 22 March 2022).
6.
UpToDate. 2022.
Stillbirth: Incidence, risk factors, etiology, and prevention 8 February 2022. URL:
UNDER
https://www.uptodate.com/contents/stil birth-incidence-risk-factors-etiology-and-prevention
(accessed 22 March 2022).
7.
AusVaxSafety. 2022.
Pfizer COVID-19 vaccine safety data - pregnant participants 21 March
2022. URL:
https://ausvaxsafety.org.au/pfizer-covid-19-vaccine-adult-formulation/pfizer-covid-
19-vaccine-safety-data-pregnant-participants (accessed 23 March 2022).
8.
Ministry of Health. 2022.
COVID-19 vaccine: Pregnancy and breastfeeding 4 February 2022.
URL:
https://www.health.govt.nz/covid-19-novel-coronavirus/covid-19-vaccines/covid-19-
vaccine-pregnancy-and-breastfeeding (accessed 23 March 2022).
INFORMATION
9.
The Royal Australian and New Zealand Col ege of Obstetricians and Gynaecologists. 2021.
RANZCOG reiterates advice on COVID-19 vaccination 24 December 2021. URL:
RELEASED
https://ranzcog.edu.au/news/ranzcog-reiterates-advice-on-covid-19-vaccination (accessed 23
March 2022).
10.
Royal Col ege of Obstetricians and Gynaecologists. 2021.
COVID-19 vaccines, pregnancy and
breastfeeding FAQs 20 December 2021. URL:
https://www.rcog.org.uk/guidance/coronavirus-
covid-19-pregnancy-and-women-s-health/vaccination/covid-19-vaccines-pregnancy-and-
breastfeeding-faqs/ (accessed 23 March 2021).
11.
Ministry of Health (Canada). 2021.
COVID-19 Vaccination Recommendations for Special
OFFICIAL
Populations 31 December 2021. URL:
https://www.ontario.ca/page/covid-19-vaccines-
pregnancy (accessed 23 March 2022).
12.
Centres for Disease Control and Prevention. 2022.
COVID-19 Vaccines While Pregnant or
Breastfeeding 3 March 2022. URL:
https://www.cdc.gov/coronavirus/2019-
ncov/vaccines/recommendations/pregnancy (accessed 23 March 2022).
13.
European Medicines Agency. 2022.
COVID-19: latest safety data provide reassurance about use
of mRNA vaccines during pregnancy 18 January 2022. URL:
Page 21 of 23
Document 1
https://www.ema.europa.eu/en/news/covid-19-latest-safety-data-provide-reassurance-about-
use-mrna-vaccines-during-pregnancy (accessed 14 February 2022).
14.
Shimabukuro TT, Kim SY, Myers TR, et al. 2021. Preliminary Findings of mRNA Covid-19
Vaccine Safety in Pregnant Persons.
New England Journal of Medicine 384(24): 2273-2282. DOI:
10.1056/NEJMoa2104983 (accessed 17 March 2022).
15.
Blakeway H, Prasad S, Kalafat E, et al. COVID-19 Vaccination During Pregnancy: Coverage and
Safety.
American Journal of Obstetrics & Gynecology
URL
:https://doi.org/10.1016/j.ajog.2021.08.007, DOI:10.1016/j.ajog.2021.08.007: DOI:
10.1016/j.ajog.2021.08.007 (accessed 17 March 2022).
16.
Theiler RN, Wick M, Mehta R, et al. 2021. Pregnancy and birth outcomes after SARS-CoV-2
vaccination in pregnancy.
American Journal of Obstetrics & Gynecology MFM 3(6): 100467. DOI:
https://doi.org/10.1016/j.ajogmf.2021.100467 (accessed 23 March 2022).
17.
Trostle ME, Limaye MA, Avtushka V, et al. 2021. COVID-19 vaccination in pregnancy: early
experience from a single institution.
American Journal of Obstetrics & Gynecology MFM 3(6):
100464. DOI:
https://doi.org/10.1016/j.ajogmf.2021.100464 (accessed 23 March 2022).
18.
Kharbanda EO, Haapala J, DeSilva M, et al. 2021. Spontaneous abortion fol owing COVID-19
1982
vaccination during pregnancy.
JAMA URL
:https://doi.org/10.1001/jama.2021.15494,
DOI:10.1001/jama.2021.15494: DOI: 10.1001/jama.2021.15494 (accessed 23 March 2022).
THE
19.
Zauche LH, Wal ace B, Smoots AN, et al. 2021. Receipt of mRNA Covid-19 Vaccines and Risk of
Spontaneous Abortion.
New England Journal of Medicine 385(16): 1533-1535. DOI:
ACT
10.1056/NEJMc2113891 (accessed 23 March 2022).
20.
Bookstein Peretz S, Regev N, Novick L, et al. 2021. Short-term outcome of pregnant women
vaccinated with BNT162b2 mRNA COVID-19 vaccine.
Ultrasound in Obstetrics & Gynecology
58(3): 450-456. DOI:
https://doi.org/10.1002/uog.23729 (accessed 23 March 2022).
21.
Kachikis A, Englund JA, Singleton M, et al. 2021. Short-term Reactions Among Pregnant and
UNDER
Lactating Individuals in the First Wave of the COVID-19 Vaccine Rol out.
JAMA Network Open
4(8): e2121310-e2121310. DOI: 10.1001/jamanetworkopen.2021.21310 (accessed 23 March
2022).
22.
Magnus MC, Gjessing HK, Eide HN, et al. 2021. Covid-19 Vaccination during Pregnancy and
First-Trimester Miscarriage.
New England Journal of Medicine 385(21): 2008-2010. DOI:
10.1056/NEJMc2114466 (accessed 16 March 2022).
23.
Wainstock T, Yoles I, Sergienko R, et al. 2021. Prenatal maternal COVID-19 vaccination and
pregnancy outcomes.
Vaccine 39(41): 6037-6040. DOI: 10.1016/j.vaccine.2021.09.012 (accessed
INFORMATION
16 March 2022).
24.
Rottenstreich M, Sela H, Rotem R, et al. 2022. Covid-19 vaccination during the third trimester
RELEASED
of pregnancy: rate of vaccination and maternal and neonatal outcomes, a multicentre
retrospective cohort study.
BJOG: An International Journal of Obstetrics & Gynaecology 129(2):
248-255. DOI:
https://doi.org/10.1111/1471-0528.16941 (accessed 16 March 2022).
25.
Lipkind HS, Vazquez-Benitez G, DeSilva M, et al. 2022. Receipt of COVID-19 Vaccine During
Pregnancy and Preterm or Smal -for-Gestational-Age at Birth — Eight Integrated Health Care
Organizations, United States, December 15, 2020–July 22, 2021.
Morbidity and Mortality Weekly
Report 71(1): 26-30. DOI:
http://dx.doi.org/10.15585/mmwr.mm7101e1 (accessed 17 March
OFFICIAL
2022).
26.
Goldshtein I, Steinberg DM, Kuint J, et al. 2022. Association of BNT162b2 COVID-19
Vaccination During Pregnancy With Neonatal and Early Infant Outcomes.
JAMA Pediatr
URL
:https://www.ncbi.nlm.nih.gov/pubmed/35142809, DOI:10.1001/jamapediatrics.2022.0001:
DOI: 10.1001/jamapediatrics.2022.0001
27.
Sadarangani M, Soe P, Shulha H, et al. 2022. Safety of COVID-19 vaccines in pregnancy: a
Canadian National Vaccine Safety (CANVAS) Network study.
medRxiv
Page 22 of 23
Document 1
URL
:https://www.medrxiv.org/content/medrxiv/early/2022/02/24/2022.02.22.22271358.ful .pdf,
DOI:10.1101/2022.02.22.22271358: 2022.02.22.22271358. DOI: 10.1101/2022.02.22.22271358
(accessed 22 March 2022).
28.
Nakahara A, Biggio JR, Elmayan A, et al. Safety-Related Outcomes of Novel mRNA COVID-19
Vaccines in Pregnancy.
Am J Perinatol, (EFirst): (accessed 21 March 2022).
29.
Aharon D, Lederman M, Ghofranian A, et al. 9900. In Vitro Fertilization and Early Pregnancy
Outcomes After Coronavirus Disease 2019 (COVID-19) Vaccination.
Obstetrics & Gynecology
URL
:https://journals.lww.com/greenjournal/Ful text/9900/In_Vitro_Fertilization_and_Early_Pregn
ancy.378.aspx, DOI:10.1097/aog.0000000000004713: 10.1097/AOG.0000000000004713. DOI:
10.1097/aog.0000000000004713 (accessed 21 March 2022).
30.
Hil son K, Clemens SC, Madhi SA, et al. 2021. Fertility rates and birth outcomes after ChAdOx1
nCoV-19 (AZD1222) vaccination.
The Lancet 398(10312): 1683-1684. DOI: 10.1016/S0140-
6736(21)02282-0 (accessed 2022/03/21).
1982
THE
ACT
UNDER
INFORMATION
RELEASED
OFFICIAL
Page 23 of 23

Document 2
Memo
Date:
22 October 2021
To:
s 9(2)(g)(ii)
, Manager, Clinical Risk Management, Medsafe
From:
s 9(2)(g)(ii)
Subject:
Pregnancy-related AEFI reports in New Zealand fol owing administration of
Comirnaty
Incident ID:
28449
Lotus Notes Location: Immunological Products & Vaccines - ISMB
1982
For your:
Action: [√] Decision: [√] Information: [√]
THE
DESCRIPTION
ACT
This memo reviews the cases describing pregnancy-related adverse events fol owing immunisation
(AEFIs) after administration of Comirnaty, summarises the information currently available on this issue,
and considers whether any further action is required.
NATURE OF THE SAFETY CONCERN
UNDER
Safety of vaccines during pregnancy
Vaccine-preventable diseases can be associated with significant morbidity and mortality in pregnant
people, foetuses, and neonates. In some cases, immune system changes during pregnancy can
increase the susceptibility of the pregnant person and foetus to certain infectious diseases and
increase the risk of serious outcomes. Vaccination can provide direct protection of pregnant women,
and can also protect the foetus and infant through placental transfer of antibodies during pregnancy
[1, 2].
INFORMATION
There are no safety concerns surrounding administration of non-live vaccines during pregnancy.
Caution around administration of live attenuated vaccines such as the measles, mumps and rubella
RELEASED
(MMR) vaccine is based on the theoretical risk of placental transfer of attenuated virus and
subsequent infection of the foetus. However, evidence of foetal harm after vaccination has not been
identified. A review of the evidence around safety of vaccination during pregnancy by the Global
Advisory Committee on Vaccine Safety found no safety concerns with influenza, tetanus toxoid,
meningococcal, MMR, poliovirus or yellow fever vaccines (Table 1) [1, 2].
OFFICIAL
Page 1 of 17

Document 2
Table 1: Summary of vaccines reviewed by the Global Advisory Committee on Safety and level of evidence concerning vaccine
safety. Source: Global Advisory Committee on Vaccine Safety. 2014. Safety of immunization during pregnancy: a review of the
evidence July 2014. URL: who.int/publications/i/item/safety-immunization-pregnancy (accessed 12 October 2021).
1982
THE
ACT
++++ Substantial evidence from RCTs, large observational studies or registries with pregnancy fol ow-up and passive surveil ance.
+++ Evidence from observational studies or registries with pregnancy follow-up and passive surveil ance.
+ + Some evidence from studies with lower power, lack of information on some relevant pregnancy outcomes, short follow-up of offspring
UNDER
or other limitations of study design and passive surveil ance.
+ Passive surveil ance data.
- No data.
Assessment of the safety of vaccination during pregnancy must be undertaken in the context of the
risks associated with infection without vaccination. The evaluation of vaccine safety is complicated by
the task of distinguishing the inherent risks of pregnancy from risks associated with vaccination. This
requires knowledge of background rates of adverse pregnancy outcomes [1].
Clinical trials usual y do not include pregnant or lactating women and newer vaccines often have
INFORMATION
limited post-market experience in pregnant women. Post-market safety studies face methodological
chal enges such as small sample sizes, limited detection of early pregnancy loss, and the long-term
RELEASED
follow-up required to detect congenital effects. Despite these chal enges, there is substantial evidence
supporting the safety of vaccination during pregnancy [1].
Risks of COVID-19 disease during pregnancy
The World Health Organisation (WHO) COVID-19 Vaccine Safety Surveil ance Manual includes the
following information on the risks of COVID-19 disease during pregnancy [3]:
‘While there is no indication that pregnant women have an increased susceptibility to infection
OFFICIAL
with SARS-CoV-2, evidence suggests that pregnant women with COVID-19 are at higher risk of
developing severe disease compared to non-pregnant women of reproductive age. As seen with
non-pregnant women, a high proportion of pregnant women have asymptomatic SARS-CoV-2
infection and severe disease is associated with recognized medical (eg, high body-mass index,
diabetes, pre-existing pulmonary or cardiac conditions) and social (eg, social deprivation,
ethnicity) risk factors. Pregnant women with symptomatic COVID‑19 appear to have an
increased risk of intensive care unit admission, mechanical ventilation and death in comparison
Page 2 of 17

Document 2
1982
THE
ACT
UNDER
INFORMATION
RELEASED
OFFICIAL

Document 2
USAGE DATA
Figure 1 shows the number of doses administered to females by age group. Figure 2 shows the
number of doses administered to females by ethnic group.
Figure 1: Vaccine doses administered to females by age group up to 21 October 2021. Source: COVID-19 Vaccination Events
Qlik app, updated 21 October 2021 (accessed 21 October 2021).
1982
THE
ACT
Figure 2: Vaccine doses administered to women of reproductive age (15-49 years) by ethnic group up to 21 October 2021.
Source: COVID-19 Vaccination Events Qlik app, updated 21 October 2021 (accessed 21 October 2021).
UNDER
INFORMATION
RELEASED
SOURCE OF SAFETY CONCERN
There have been reports of AEFIs in pregnant women administered Comirnaty in New Zealand.
OFFICIAL
This report includes AEFI reports that have been coded with terms under the pregnancy, puerperium
and perinatal conditions system organ class (SOC). The terms reported as of 21 October 2021 are
spontaneous abortion, exposure during pregnancy, foetal hypokinesia and congenital abnormality.
There have been 13 cases of spontaneous abortion in New Zealand reported following administration
of Comirnaty, as of 21 October 2021. s 9(2)(a)
Page 4 of 17
Document 2
Three cases were people aged under 20-29 years, eight cases were in people aged 30-39 years, and
two cases were in people aged 40-49 years. s 9(2)(a)
The remaining three cases describe exposure during pregnancy/dizziness, palpitations/foetal
hypokinesia, and congenital abnormalities.
Annex 1 contains detailed information about the cases.
s 9(2)(f)(iv), s 9(2)(a)
1982
THE
ACT
UNDER
s 9(2)(a)
INFORMATION
RELEASED
OFFICIAL
Page 5 of 17
Document 2
s 9(2)(a)
1982
THE
ACT
UNDER
INFORMATION
RELEASED
OFFICIAL
Page 6 of 17
Document 2
s 9(2)(a)
REVIEW OF THE AVAILABLE INFORMATION
New Zealand data sheet and international product information
Section 4.6 of the New Zealand data sheet includes the following information relating to use in
pregnancy:
‘There is limited experience with use of COMIRNATY in pregnant women. Animal studies do not
indicate direct or indirect harmful effects with respect to pregnancy, embryo/fetal development,
parturition or post-natal development (see Fertility). Administration of COMIRNATY in
pregnancy should only be considered when the potential benefits outweigh any potential risks
for the mother and fetus.’
The United Kingdom summary of product characteristics and Australian product information are
1982
identical to the New Zealand data sheet.
The Canadian product monograph states:
THE
‘The safety and efficacy of COMIRNATY in pregnant women have not yet been established.
ACT
Animal studies do not indicate direct or indirect harmful effects with respect to pregnancy,
embryo/ fetal development, parturition, or post-natal development (see 16 NON-CLINICAL
TOXICOLOGY).’
The United States prescribing information states:
UNDER
‘Al pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the US general
population, the estimated background risk of major birth defects and miscarriage in clinical y
recognized pregnancies is 2% to 4% and 15% to 20%, respectively. Available data on Pfizer-
BioNTech COVID-19 Vaccine administered to pregnant women are insufficient to inform
vaccine-associated risks in pregnancy.
In a reproductive and developmental toxicity study, 0.06 mL of a vaccine formulation containing
the same quantity of nucleoside-modified messenger ribonucleic acid (mRNA) (30 mcg) and
other ingredients included in a single human dose of Pfizer-BioNTech COVID-19 Vaccine was
INFORMATION
administered to female rats by the intramuscular route on four occasions: 21 and 14 days prior
to mating, and on gestation days 9 and 20. No vaccine-related adverse effects on female
RELEASED
fertility, fetal development, or postnatal development were reported in the study.’
s 9(2)(f)(iv), s 9(2)(a)
OFFICIAL
Page 7 of 17
Document 2
s 9(2)(a), s 9(2)(f)(iv)
1982
THE
ACT
UNDER
INFORMATION
RELEASED
OFFICIAL
Page 8 of 17
Document 2
s 9(2)(a), s 9(2)(f)(iv)
1982
THE
ACT
UNDER
INFORMATION
RELEASED
OFFICIAL
Page 9 of 17
Document 2
s 9(2)(a), s 9(2)(f)(iv)
1982
THE
ACT
UNDER
s 9(2)(f)(iv), s 9(2)(b)(ii)
INFORMATION
Communications from international regulators, organisations and government departments
The New Zealand Ministry of Health, Royal Australian and New Zealand Col ege of Obstetricians and
RELEASED
Gynaecologists (RANZCOG), Canadian Ministry of Health, Royal Col ege of Obstetricians and
Gynaecologists (UK) and Centres for Disease Control and Prevention (US) have issued statements in
support of routine COVID-19 vaccination of pregnant people. [5-9]
The WHO has published the fol owing guidance on COVID-19 vaccination during pregnancy: [3]
‘At present (April 2021), the WHO Strategic Advisory Group of Experts on Immunization (SAGE)
recommends that pregnant women can receive COVID‑19 vaccine if the benefits of vaccination
OFFICIAL
outweigh the potential risks, such as occupational activities with unavoidable high risk of
exposure, and pregnant women with co-morbidities which place them in a high-risk group for
severe COVID‑19 disease. In other words, vaccination for pregnant women should be considered
on a case by case basis after consultation between the woman and her health care provider. To
help pregnant women decide, they should be provided with information about the risks of
COVID-19 in pregnancy, the likely benefits of vaccination in the local epidemiological context,
and the current limitations of the safety data for the vaccines in pregnant women.
Page 10 of 17
Document 2
As more data become available these guidelines wil be updated. Routine testing for pregnancy
before COVID‑19 vaccination is not recommended.’
The WHO has also issued guidance on communicating with pregnant and lactating women during
COVID-19 vaccination sessions. [10]
Literature
Several studies describing the safety of COVID-19 vaccination during pregnancy are summarised
below. A full list of studies identified relating to safety of COVID-19 vaccines in pregnancy is included
in Annex 2.
Shimabukuro et al. 2021. Preliminary Findings of mRNA Covid-19 Vaccine Safety in Pregnant Persons
[11]
This study aimed to characterise the safety of mRNA vaccines (ie, Comirnaty and Spikevax) in pregnant
people in the United States. Data from the ‘v-safe after vaccination health checker’ surveil ance
system, the v-safe pregnancy registry, and the Vaccine Adverse Event Reporting System (VAERS)
1982
obtained between December 2020 and February 2021 were evaluated.
The v-safe surveil ance system is a voluntary smartphone-based active surveil ance system, which
THE
sends links to online surveys immediately after vaccination through to one year after vaccination. V-
safe is used to identify people who reported being pregnant, who are then invited to join the v-safe
ACT
pregnancy registry. Detailed medical information is col ected about the participants in the pregnancy
registry via telephone interviews.
Adverse reaction reports in pregnant people from the spontaneous reporting system (VAERS) were
also reviewed for the study. Reporting of pregnancy-related complications resulting in hospitalisation
and congenital abnormalities is required of healthcare professionals under the conditions of the
UNDER
Emergency Use Authorisations for COVID-19 vaccines in the United States.
V-safe data was used to compare the reported proportions of local and systemic reactogenicity
between pregnant and non-pregnant people. In the v-safe pregnancy registry, the outcomes of
completed pregnancies were evaluated. The outcomes reported included pregnancy loss
(spontaneous abortion and stil birth) and neonatal outcomes (preterm birth, congenital anomalies,
small size for gestational age, and neonatal death).
During the study period for the interim analysis, 35,691 v-safe participants identified as pregnant. The
INFORMATION
most frequently reported reactogenicity symptoms were injection-site pain, fatigue, headache, and
myalgia, which were reported more frequently after the second dose. The reactogenicity profile was
RELEASED
similar between pregnant and non-pregnant people. Nausea and vomiting was reported in a slightly
higher proportion of pregnant people.
As of March 30 2021, there were 3,958 pregnant people who were vaccinated during the study period
enrol ed into the v-safe pregnancy registry, of whom 94% identified as healthcare personnel. There
were 827 participants with a completed pregnancy of which 712 (86.1%) resulted in a live birth, 104
(12.6%) resulted in a spontaneous abortion, 1 (0.1%) resulted in still-birth and 10 (1.2%) resulted in
OFFICIAL
other outcomes (induced abortion and ectopic pregnancy). Of the 104 spontaneous abortions, 96
(92.3%) occurred during the first trimester of pregnancy. The adverse outcomes in 724 live-born
infants were pre-term birth (9.4%), small size for gestational age (3.2%) and major congenital
abnormalities (2.2%). There were no neonatal deaths. None of the reports of major congenital
abnormalities were associated with vaccination during the periconception period or first trimester.
Proportions of adverse pregnancy and neonatal outcomes were similar to published incidences in the
literature (table 2).
Page 11 of 17

Document 2
Table 2: Pregnancy loss and neonatal outcomes in published studies and v-safe pregnancy registry participants. Source:
Shimabukuro TT, Kim SY, Myers TR, et al. 2021. Preliminary Findings of mRNA Covid-19 Vaccine Safety in Pregnant Persons.
New England Journal of Medicine 384(24): 2273-2282. DOI: 10.1056/NEJMoa2104983.
1982
THE
ACT
There were 221 reports retrieved from VAERS describing vaccination of pregnant people. Of these,
155 (70.1%) described adverse events not specific to pregnancy, and 66 (29.9%) described adverse
UNDER
events relating to the pregnancy or neonate. There were 46 cases of spontaneous abortion, of which
37 were during the first trimester and two were during the second trimester. In seven cases, the
trimester was unknown. There were three reports each of stil birth, premature rupture of membranes,
and vaginal bleeding, and no reports of congenital abnormalities.
The authors noted a number of limitations in the study, including:
• Comparison of proportions of adverse pregnancy and neonatal outcomes are limited by
differences in the populations studied and are intended provide a crude sense only of any
unexpected signals
INFORMATION
• V-safe participants self-reported their pregnancy status, and data describing local and systemic
reactogenicity may be subject to misclassification
RELEASED
• The survey data does not al ow assessment of time to onset or duration of reactogenicity
symptoms
• The data are preliminary, based on a smal sample size, and mostly describe outcomes
following third trimester vaccination
• The follow-up period was too short to capture first trimester vaccination and subsequent
congenital abnormality outcomes
OFFICIAL
• The proportions of spontaneous abortion may not reflect true post-vaccination proportions
because participants might have been vaccinated after the period of greatest risk in the first
trimester, and early pregnancy losses may have been undetected
• At the time the study was conducted, most of the pregnancies involving vaccination during the
first and second trimesters were ongoing
• The study uses data reported by participants, with limited information on other risk factors for
adverse pregnancy and neonatal outcomes
• VAERS is subject to the limitations of passive surveillance
Page 12 of 17
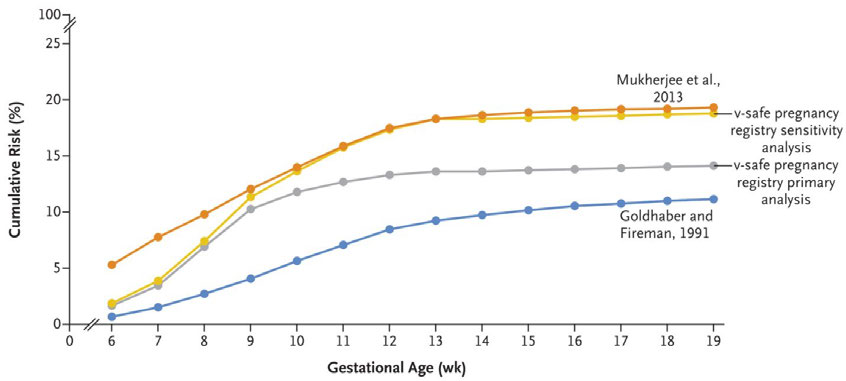
Document 2
• The total number of vaccine doses administered to pregnant women is unknown and creates
limitations to estimating rates of adverse pregnancy and neonatal outcomes.
This preliminary data does not indicate any safety signals in pregnant people administered mRNA
vaccines. Continued monitoring is needed to add to these results.
Zauche et al. 2021. Receipt of mRNA Covid-19 Vaccines and Risk of Spontaneous Abortion [12]
This research letter uses data from the Centres for Disease Prevention (CDC) v-safe pregnancy registry
to determine the cumulative risk of spontaneous abortion from 6 to less than 20 weeks of gestation.
A total of 2456 participants were included in the study, of which 2022 reported ongoing pregnancies
at 20 weeks and 165 reported a spontaneous abortion after six weeks of gestation and before 20
weeks of gestation. The remainder were not fol owed up at 20 weeks gestation or later, or reported
another pregnancy outcome before 20 weeks gestation.
The cumulative risk of spontaneous abortion was calculated according to gestational week using life
table methods. Another analysis was age-standardized with the use of data on the risk of spontaneous
abortion according to maternal age group. A sensitivity analysis was conducted that assumed that 65
1982
participants with ongoing pregnancy during the first trimester that could not be reached for second
trimester follow-up had a spontaneous abortion.
THE
This data was compared to historical cohorts that represent the upper and lower ranges of
ACT
spontaneous abortion risk. The cumulative risks calculated in this study fal within this range (figure 6).
Figure 6: Cumulative risk of spontaneous abortion in the v-safe COVID-19 vaccine pregnancy registry and in two historical
cohorts. Data from Mukherjee et al were presented as race-specific rates and are provided here for white women to maximize
comparability with the v-safe pregnancy registry. Source: Zauche et al. 2021. Receipt of mRNA Covid-19 Vaccines and Risk of
Spontaneous Abortion. New England Journal of Medicine 385(16): 1533-1535. DOI: 10.1056/NEJMc2113891.
UNDER
INFORMATION
RELEASED
Blakeway et al. 2021. COVID-19 Vaccination During Pregnancy: Coverage and Safety [13]
This cohort study aimed to investigate the uptake and safety of COVID-19 vaccination among
pregnant women in the United Kingdom. The cohort was derived from those who gave birth at St
George’s University Hospitals National Health Service Foundation Trust, London, United Kingdom,
OFFICIAL
between March 1, 2020, and July 4, 2021. The primary outcome in this study was vaccine uptake, and
secondary outcomes were perinatal safety outcomes, including stil birth, preterm birth, foetal and
congenital abnormalities, and intrapartum complications. People who received a COVID-19 vaccine
(Comirnaty, Spikevax or AstraZeneca) were compared to a propensity score-matched cohort of people
with balanced propensity scores.
Page 13 of 17
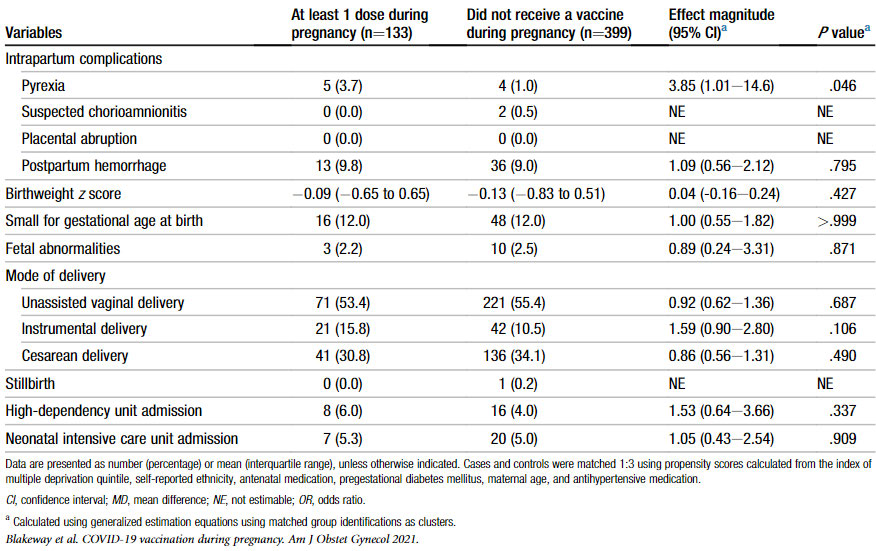
Document 2
Data were available for 1328 pregnant women, of whom 140 received at least one dose of a COVID-19
vaccine. Of the vaccinated group, 127 (90%) received an mRNA vaccine. The vaccination was
administered during the third trimester in 85.7% of people and during the second trimester in 14.3%
of people.
The rates of adverse pregnancy outcomes were similar between vaccinated and unvaccinated people
(table 3). A statistical y significant increase in intrapartum pyrexia lost this significance when people
with antenatal COVID-19 infection were excluded.
Table 3: Pregnancy outcomes among propensity score-matched women who received at least 1 dose of the COVID-19 vaccine.
Source: Blakeway et al. COVID-19 Vaccination During Pregnancy: Coverage and Safety. American Journal of Obstetrics &
Gynecology. DOI:10.1016/j.ajog.2021.08.007 (accessed 2 September 2021).
1982
THE
ACT
UNDER
INFORMATION
The authors noted the fol owing limitations of the study:
• Median time to birth after vaccination was one month
RELEASED
• None of the participants were vaccinated in the first trimester of pregnancy and only 15% were
vaccinated in the second trimester
• People without vaccination records were not included in the study, which may have led to
selection bias
• The study was not sufficiently powered to detect smal effect sizes and 95% confidence
intervals were wide.
OFFICIAL
This study contributes to the evidence of safety of mRNA vaccination during pregnancy, particularly
during the third trimester.
Bleicher et al. 2021. Early exploration of COVID-19 vaccination safety and effectiveness during
pregnancy: interim descriptive data from a prospective observational study [14]
This interim observational study analysis examines short-term pregnancy outcomes reported after
vaccination of pregnant women in Israel. Vaccinated and non-vaccinated women were recruited
through social media and information was col ected using a questionnaire. The primary outcomes
Page 14 of 17
Document 2
were composite pregnancy complications such as vaginal bleeding, pregnancy loss during first
trimester, pregnancy loss during second trimester, gestational diabetes, premature birth, premature
contractions, and foetal growth restriction. Secondary outcomes were vaccine side effects, COVID-19
infection, uptake of vaccination and reasons for refusal of vaccination.
The characteristics of the vaccinated and non-vaccinated women completing the initial questionnaire
were somewhat similar, but there was a higher rate of previous pregnancy loss in the unvaccinated
group. It is unclear if there was differential loss to follow-up.
The initial and follow-up questionnaires were answered by 326 women, of whom 202 were vaccinated.
Of the vaccinated group, 17.8% were vaccinated in the first trimester, 54.5% were vaccinated in the
second trimester, and 27.7% were vaccinated in the third trimester.
The rate of composite pregnancy complications was similar between vaccinated and non-vaccinated
group (15.8% vs 20.1%). First trimester pregnancy loss was reported by two people in the vaccinated
group and one in the non-vaccinated group.
This analysis is limited due to self-reporting of outcomes and short-term follow-up. The numbers of
1982
reported adverse pregnancy outcomes were too smal to make meaningful comparisons. The study is
ongoing.
THE
Trostle et al. 2021. COVID-19 vaccination in pregnancy: early experience from a single institution [15]
ACT
This study describes pregnancy outcomes for 424 people at a single medical centre who were
vaccinated against COVID-19 during pregnancy. Of these 424 people, 29.2% were first vaccinated
during the first trimester, 45.5% during the second trimester and 25.2% during the third trimester.
Comirnaty was administered to 78.3% of participants and Spikevax was administered to 21.7% of
participants.
UNDER
Nine people had spontaneous abortions, of which eight were during the first trimester and one was
during the second trimester. The rate of spontaneous abortion among women vaccinated during the
first trimester was 6.5%. Of the women included, 85 delivered liveborn infants. There were no
stillbirths. The rate of pre-term birth was 5.9%. Of the liveborn infant, 12.2% were smal for gestational
age. No concerning trends were identified. The study was limited by a smal sample size and lack of a
matched control group.
PUBLIC INTEREST
INFORMATION
There is significant public concern about the safety of COVID-19 vaccination during pregnancy, likely
due in part to unsubstantiated claims of fertility concerns associated with COVID-19 vaccination.
RELEASED
EXPERT ADVICE
It is recommended that the COVID-19 Vaccine Independent Safety Monitoring Board (CV-ISMB) is
updated on the spontaneous reporting data in pregnant women that is available so far.
CONCLUSIONS AND PROPOSED ACTIONS
OFFICIAL
In general, there are no safety concerns associated with the use of non-live vaccines in pregnancy.
Caution around live attenuated vaccines is based on the theoretical risk of placental transfer of
attenuated virus and subsequent infection of the foetus. However, evidence of foetal harm after
vaccination has not been identified [1, 2].
Pregnant women with symptomatic COVID‑19 appear to have an increased risk of intensive care unit
admission, mechanical ventilation and death in comparison with non-pregnant women of
reproductive age, and may also be at increased risk of preterm birth [3].
Page 15 of 17
Document 2
There have been 13 reports of spontaneous abortion in New Zealand fol owing administration of
Comirnaty to pregnant people, s 9(2)(a)
. Spontaneous abortion
is common, occurring in around 10% of clinical y recognised pregnancies. This risk increases with age
[4]. The reports received to date do not highlight any concerning trends, although they contain limited
information.
The published literature to date has not highlighted safety concerns around COVID-19 vaccination of
pregnant women, although results are preliminary.
Reports of pregnancy related AEFIs in New Zealand should continue to be closely monitored.
RECOMMENDATIONS
It is recommended that:
1.
The CV-ISMB is updated on the spontaneous reporting data in pregnant
Yes
women
1982
2.
This topic should continue to be monitored through routine
Yes
pharmacovigilance activities
THE
R
ACT
EFERENCES
1.
Global Advisory Committee on Vaccine Safety. 2014.
Safety of immunization during pregnancy:
a review of the evidence July 2014. URL: https://www.who.int/publications/i/item/safety-
immunization-pregnancy (accessed 12 October 2021).
2.
Ministry of Health. 2020.
Immunisation Handbook 2020 September 2020. URL:
UNDER
https://www.health.govt.nz/our-work/immunisation-handbook-2020 (accessed 12 October
2021).
3.
World Health Organisation. 2021.
COVID-19 Vaccines: safety surveillance manual. Module on
safety surveillance of COVID-19 vaccines in pregnant and breastfeeding women
(WHO/MHP/RPQ/PVG/2021.1) 2021. URL: https://www.who.int/publications/i/item/WHO-
MHP-RPQ-PVG-2021.1 (accessed 15 October 2021).
4.
UpToDate. 2021.
Pregnancy loss (miscarriage): Terminology, risk factors, and etiology 24
September 2021. URL: https://www.uptodate.com/contents/pregnancy-loss-miscarriage-
INFORMATION
terminology-risk-factors-and-etiology (accessed 21 October 2021).
5.
Gynaecologists RAaNZCoOa. 2021.
COVID-19 Vaccination in Pregnant and Breastfeeding
RELEASED
Women and those planning pregnancy 18 August 2021. URL:
https://ranzcog.edu.au/statements-guidelines/covid-19-statement/covid-19-vaccination-
information (accessed 19 October 2021).
6.
Health Mo. 2021.
COVID-19 vaccine: Pregnancy and breastfeeding 13 October 2021. URL:
https://www.health.govt.nz/our-work/diseases-and-conditions/covid-19-novel-
coronavirus/covid-19-vaccines/covid-19-vaccine-health-advice/covid-19-vaccine-pregnancy-
and-breastfeeding (accessed 19 October 2021).
OFFICIAL
7.
Ministry of Health (Canada). 2021.
COVID-19 Vaccination Recommendations for Special
Populations 29 September 2021. URL:
https://www.health.gov.on.ca/en/pro/programs/publichealth/coronavirus/docs/vaccine/COVID
-19 vaccination rec special populations.pdf (accessed 19 October 2021).
8.
Gynaecologists RCoOa. 2021.
COVID-19 vaccines, pregnancy and breastfeeding 11 October
2021. URL: https://www.rcog.org.uk/en/guidelines-research-services/coronavirus-covid-19-
Page 16 of 17
Document 2
pregnancy-and-womens-health/covid-19-vaccines-and-pregnancy/covid-19-vaccines-
pregnancy-and-breastfeeding/ (accessed 19 OCtober 2021).
9.
Centres for Disease Control and Prevention. 2021.
COVID-19 Vaccines While Pregnant or
Breastfeeding 7 October 2021. URL: https://www.cdc.gov/coronavirus/2019-
ncov/vaccines/recommendations/pregnancy.html (accessed 19 October 2021).
10.
World Health Organisation. 2021.
Health worker communication for COVID-19 vaccination flow
diagram 13 May 2021. URL: https://www.who.int/publications/m/item/health-worker-
communication-for-covid-19-vaccination-flow-diagram (accessed 15 October 2021).
11.
Shimabukuro TT, Kim SY, Myers TR, et al. 2021. Preliminary Findings of mRNA Covid-19
Vaccine Safety in Pregnant Persons.
New England Journal of Medicine 384(24): 2273-2282. DOI:
10.1056/NEJMoa2104983 (accessed 21 October 2021).
12.
Zauche LH, Wal ace B, Smoots AN, et al. 2021. Receipt of mRNA Covid-19 Vaccines and Risk of
Spontaneous Abortion.
New England Journal of Medicine 385(16): 1533-1535. DOI:
10.1056/NEJMc2113891 (accessed 21 October 2021).
13.
Blakeway H, Prasad S, Kalafat E, et al. COVID-19 Vaccination During Pregnancy: Coverage and
Safety.
American Journal of Obstetrics & Gynecology
1982
URL:https://doi.org/10.1016/j.ajog.2021.08.007, DOI:10.1016/j.ajog.2021.08.007 (accessed 21
October 2021).
THE
14.
Bleicher I, Kadour-Peero E, Sagi-Dain L, et al. 2021. Early exploration of COVID-19 vaccination
safety and effectiveness during pregnancy: interim descriptive data from a prospective
ACT
observational study.
Vaccine 39(44): 6535-6538. DOI:
https://doi.org/10.1016/j.vaccine.2021.09.043 (accessed 21 October 2021).
15.
Trostle ME, Limaye MA, Avtushka V, et al. 2021. COVID-19 vaccination in pregnancy: early
experience from a single institution.
American Journal of Obstetrics & Gynecology MFM 3(6):
100464. DOI: https://doi.org/10.1016/j.ajogmf.2021.100464 (accessed 21 October 2021).
UNDER
INFORMATION
RELEASED
OFFICIAL
Page 17 of 17
Document Outline
- 1. COVID-19 vaccination in pregnancy memo (28963)_to ISMB
- Description
- Nature of the Safety Concern
- Products
- Indications
- Information in local and international product information
- Source of Safety Concern
- Review of the available information
- Spontaneous reporting data in New Zealand
- Solicited reporting in Australia
- Recommendations from local and international bodies
- Literature
- Literature on pregnancy outcomes
- Magnus et al, 2021. COVID-19 vaccination during pregnancy and first-trimester miscarriage [22]
- Wainstock et al, 2021. Prenatal maternal COVID-19 vaccination and pregnancy outcomes [23]
- Rottenstreich et al, 2021. Covid-19 vaccination during the third trimester of pregnancy: rate of vaccination and maternal and neonatal outcomes, a multicentre retrospective cohort study [24]
- Lipkind et al, 2022. Receipt of COVID-19 vaccine during pregnancy and preterm or small-for-gestational-age at birth — eight integrated health care organizations, United States, December 15, 2020-July 22, 2021 [25]
- Goldshtein et al, 2022. Association of BNT162b2 COVID-19 vaccination during pregnancy with neonatal and early infant outcomes [26]
- Literature on general safety profile in pregnant people
- Literature on fertility
- Periodic Benefit Risk Evaluation Reports (PBRERs)
- Expert Advice
- Conclusion and Proposed Actions
- Recommendations
- References
- 2. Pregnancy-related AEFI reports in New Zealand following administration of Comirnaty (#28449) marked for redaction - Copy

























