

23 August 2024
Carl Berryman
[FYI request #26354 email]
Tēnā koe Carl
Your request for official information, reference: HNZ00049846
Thank you for your email on 27 May 2024, extended 25 June 2024, asking Health New
Zealand | Te Whatu Ora for the following under the Official Information Act 1982 (the Act):
Your response to my two requests for information is extroadinary, considering your
response letter to me clearly states; “The pilot initiative referenced in your request was
established and managed by the Ministry of Health, prior to the creation of Health New
Zealand in July 2022”.
Your letter confirms the existence of such a 'Pilot' iniative/program, hence I am at a
loss to understand how can you say; “we do not hold any information within the scope
of your request”.
The existence of the information I have requested is also evidenced in a previous OIA
response. I refer to minutes from the Covid-19 Vaccine and Immunisation Programme
Steering Group of Tuesday, 10 August 2021, chaired by Dr Ashley Bloomfield –
Agenda item 10b, update given by Rachel Mackay, which states ….... “The two pilot
employers (Mainfreight, Fonterra) wil both have completed their first round by 11
August. The Ministry wil debfrief with both on the learnings from these pilots.”
I struggle to understand that Health NZ and/or the Ministry of Health now claim that
neither party do not hold any information on this 'Pilot' initiative/program.
I need to point out that Health NZ is regulated by the same NZ laws and contains the
same policies & staff as the Ministry of Health. It’s therefore reasonable to expect full
transparency about this publicly-funded 'Pilot' initiative/program. A simple keyword
search of relevant communications at that time would assist in your search for
information.
I am hopeful that I am not forced into the position of having to complain to the
Ombudsman. Please supply the information requested, as the information does exist.
I also need to reference the details shown below for my second request on information
regarding vaccines sent to and administered in workplaces. This was most likely
facilitated through the 'Pilot' initiative/program.
The minutes discuss the worker and workplace vaccination program here:
https://aus01.safelinks.protection.outlook.com/?url=https%3A%2F%2Ffyi.org.nz%2Fre
quest%2F15656%2Fresponse%2F73774%2Fattach%2F5%2FH202116597%2520Doc
ument%25201.pdf&data=05%7C02%7CHNZOIA%40tewhatuora.govt.nz%7C8af37ae2
a6414eba914208dc7b91c52c%7Cbed4da513cdb4d0dbaf8fb80d53268e3%7C0%7C0
%7C638521113239716668%7CUnknown%7CTWFpbGZsb3d8eyJWIjoiMC4wLjAwMD
AiLCJQIjoiV2luMzIiLCJBTiI6Ik1haWwiLCJXVCI6Mn0%3D%7C0%7C%7C%7C&sdata
=Q0TjEgZdDa5Vq7kDl7%2B6jd6LS%2FdpFIQT0r%2F6YWfu6Do%3D&reserved=0
Please also provide to me all the information sought regarding the workplace vaccine
funded program of work.

On 18 July 2024, we communicated our decision on your request.
We sincerely apologise for the delay in responding to you.
Response
Our earlier correspondence (ref: HNZ00043708) described how the pilot programme in
question was managed by the Ministry of Health, prior to the existence of Health NZ.
While Health NZ now ‘owns’ your request on the basis that this subject area is now more
closely connected to our functions, because the pilot was overseen by the Ministry at
that time (back in 2021), we still do not hold any information within scope.
However, we have consulted our Ministry colleagues, who searched their records again
and we are pleased to advise some documentation has been found in scope of your
request.
We are releasing this information do you in full. These documents are outlined in the
table below and enclosed as
Annex One:
Title
Doc 1
COVID-19 messages for Business NZ meeting - 9 July 2021
Doc 2
BioNTech/Pfizer COVID-19 Vaccine and Immunisation Programme -
Planning blueprint: Workplace sites
Doc 3
Public Service Workplace Vaccinations Workshop
Doc 4
Pfizer Covid-19 Vaccination and Immunisation Programme -
Workplace model lessons learnt
Please note, the Ministry also undertook a wider IT search of their systems to locate
anything else that may be in scope. Their search used keyword(s) including but not
limited to “COVID-19 vaccination workplace programme” and “COVID-19 workplace
vaccination” and involved key Ministry staff between the dates of 1 July 2021 and
30 September 2021. This search identified over 7,500 results.
While we have been advised many of these items are emails (and possibly only
administrative in nature), some of these results may contain documentation also within
scope of your request. However, filtering through this number of results would require
the Ministry to divert personnel from their core duties and allocate extra time to complete
this task. This diversion of these resources would impair their ability to carry out their
other core functions
For this reason, Health NZ (on behalf of the Ministry) is refusing any additional
documentation that may be in scope of your request under section 18(f) of the Act, as it
involves substantial collation and research.
We have considered whether fixing a charge for the supply of the information or
extending the timeframe for response would enable us to respond, but we do not
consider that either option would remove the impact that supplying the information would
have on the Ministry’s other operations. We also hope that the information provided with
this response goes at least some way towards meeting your expectations as well as our
obligations under the Act.
How to get in touch
If you have any questions, you can contact us at
[email address].



As you know, you still have the right to make a complaint to the Ombudsman if you are not
happy with this latest response. Information about how to do this is available at
www.ombudsman.parliament.nz or by phoning 0800 802 602.
As this information may be of interest to other members of the public, Health NZ may
proactively release a copy of this response on our website. Al requester data, including your
name and contact details, wil be removed prior to release.
Nāku iti noa, nā
Danielle Coe
Manager (OIAs) – Government Services
Office of the Chief Executive
TeWhatuOra.govt.nz
Health NZ, PO Box 793,
Wel ington 6140, New Zealand
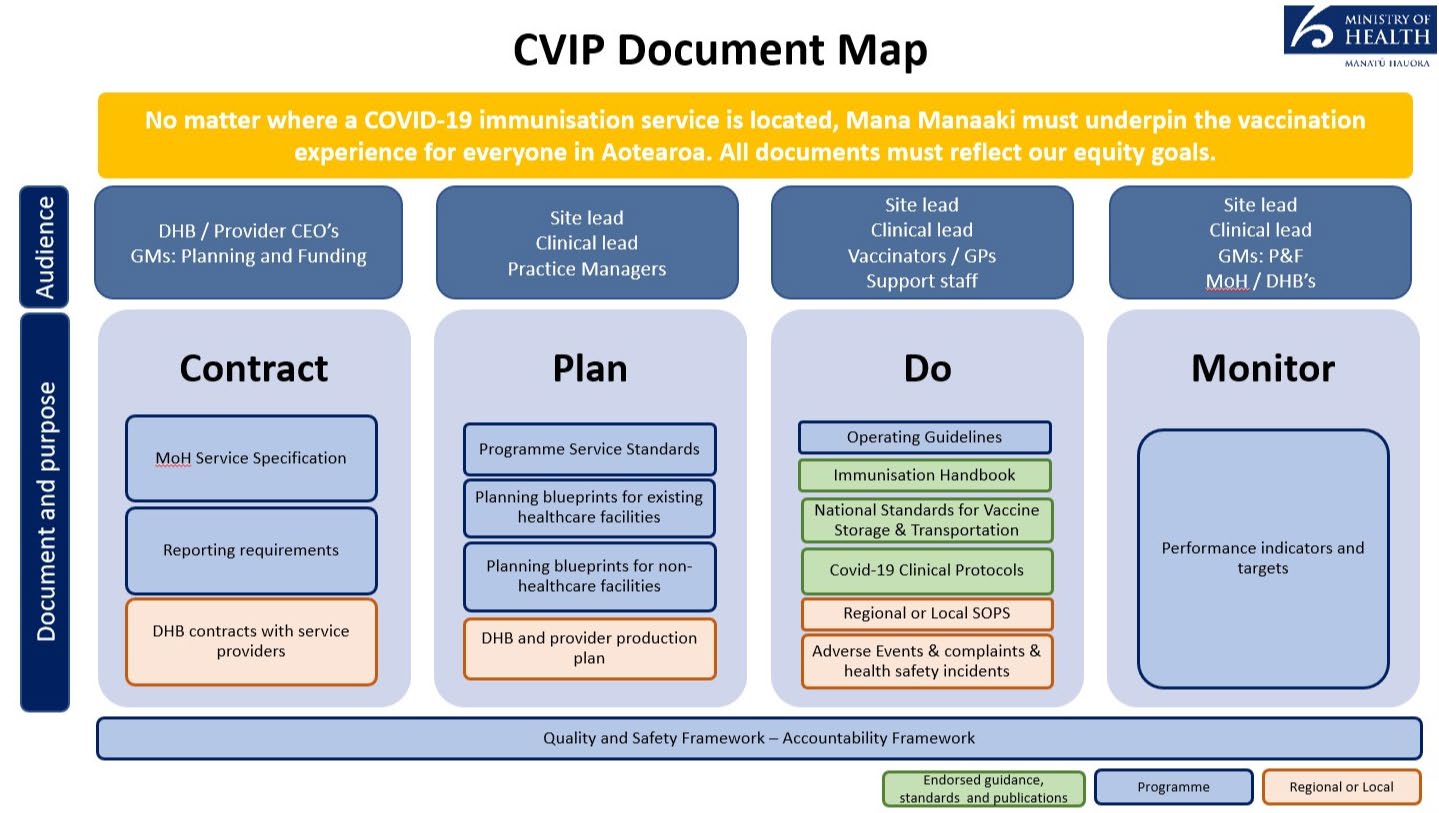
Document 1
COVID-19 messages for Business NZ meeting - 9 July 2021
Current stats
• The Ministry is leading the rollout of the Pfizer vaccine to ensure all people in Aotearoa New
Zealand are vaccinated against COVID-19.
• The cumulative number of doses administered by the end of Thursday was 1.3M doses
including over 500k people that have been ful y vaccinated.
• Whakarongorau services (Healthline) supporting the vaccine rol out. This week for example
they’ve responded to nearly 10,000 calls and make outbound calls as well.
• Al DHBs have been migrated to the National Immunisation Booking System (NIBS), which
nearly half a million active future bookings at over 100 vaccination sites.
1982
• Average time between first and second dose – 24.80 days. Minimum is 21 days.
Operations
Act
• Implementation largely devolved to DHBs and commissioned third parties e.g. PHOs. Work
with DHBs on production plans which are published on MoH website.
• Group 4 – general population can be invited from late July. Approx 2 mil ion people.
• To achieve this goal, a large workforce is required to administer the vaccines along with a
range of site options that are convenient to al population groups. Workplace vaccinations is
one of these site options.
• Three phases of workplace vaccinations:
o Have been supporting workplace vaccinations for populations in Groups 1 and 2 of
the Sequencing Framework (eg MIQ, border works – NZDF, Police; frontline – FENZ;
Corrections – staff and people in Corrections care)
Information
o ‘test sites’ - Fonterra, Mainfreight and a couple of other organisations coming in
behind these two – applying lessons learned.
o EOI process to identify interested/ eligible parties – issue today, responses due late
July, implement Sept-Nov. Trying to indicate alignment with age banding.
EOI
[email address] Official
• The Ministry is seeking expressions of interest (EOI) from employers/workplaces and
potential vaccination providers to identify the eligible demand for this site option. This EOI
the
process does not guarantee that individual employers/workplaces or vaccination providers
wil be able to participate in the programme
• Seeking joint EOIs from employers and vaccination providers. Where employers or providers
are interested but have not been able to submit a joint application, the Ministry wil
consider these individual expressions of interest in the second instance.
under
• The COVID-19 vaccination programme requires the parties to use the online National
Immunisation Booking System (NIBS) and online COVID-19 Immunisation Register (CIR) during
the vaccination event, requiring workplaces to have excellent IT connectivity.
• Workplaces must either
o be considered one of the largest workplaces/employers (by size of workforce) and
have enough workers per site to be vaccinated. As a guide, an indication is workplaces
with 1,000+ employees, with the ability to vaccinate several hundred staff per site, or
Released ofor smaller workplaces/employers – support a DHBs’ equity goals of targeting
workplaces with high Māori, Pacific or ethnic populations and those harder to reach
(e.g. due to rurality or shift work). Note even for smaller workplaces/employers –
there is a minimum requirement of 70 vaccinations per site per day
and
1
COVID-19 messages for Business NZ meeting - 9 July 2021
o have had a successful vaccine programme previously delivered onsite
o be able to provide staff to undertake specified roles and responsibilities as outlined in
the
Workplace Model Planning Blueprint – particularly the logistical tasks to support
worker engagement, recall processes, and cultural and religious safety.
o Eligibility criteria
Workplace Model Planning Blueprint CVIP Operating Guidelines
Timetable
Date
Milestone
9 July 2021
EOI issued
12pm on 21 July 2021
Closing date for respondent queries or clarifications
12pm on 23 July 2021
Closing date for EOI
1982
How companies can support staff
While we encourage workplaces to express interest in this programme, they should also consider
other ways they could support workers getting a vaccine, such as allowing workers to be vaccinated
Act
during work hours and sharing accurate and reliable information from trusted sources –
Health.govt.nz and Covid19.govt.nz.
Workforce
• 8,908 vaccinators have completed vaccine training and 3,787 vaccinators have been active in
the programme.
• Workforce to scale up – actively training vaccinator workforce plus bring on board existing
Information
medical professionals who undertake vaccinations – GPs, pharmacists, occupational health.
• Reminder that most organisations have no legal authority to mandate vaccination in their
workers unless it is included in their signed terms and conditions, but a strong
communication programme is encouraged, with reminders to rely on recognised sources.
Supply
Official
• New Zealand is already receiving significant deliveries of the Pfizer vaccine and has secured
enough doses of the Pfizer vaccine for the population of New Zealand and our Pacific
neighbours, in 2021. 10 million doses.
• Pfizer is meeting delivery commitments.
the
• Weekly deliveries arrive in Akld from Belgium. Normally lands early Tues morning.
• Going to deliver 4 million doses in July – Sept quarter. 1 mil ion doses on schedule to be
delivered in July. 150k this week (arrived ahead of schedule on last week, similar next week,
fol owed by 350k doses in each of the last two weeks of the month)
• Distributed al stock and need to rebuild stock on hand at wholesalers in Akld.
under
Logistics
• Vaccine arrives in vials – approx. 6 doses per vial, and in boxes of 195 vials – so ~1000 doses.
• Shipped at -70. Once out of -70 can’t be stored back in freezers.
• Can be stored at normal cold chain (2-8) for 31 days. Was 5 days – which was very tricky.
• Can split packs into 15’s (90 doses) or 5’s (30 doses).
• Daily orders received through log team, HCL calculate how many trays to remove and
allocate – occasional y need to send slightly more/less depending on how we split the trays.
Released
• Distributed by air and road courier with security, GPS trackers and temperature loggers.
Road freight upper North Island; airfreight at night to South Island and then return via
Palmerston North for lower North Island. Al sites have stock by late morning including
remote sites.
2
COVID-19 messages for Business NZ meeting - 9 July 2021
• Mixed model for local distribution – some use DHB hospital pharmacy, others use direct
distribution to facilities (where vaccine is stored) and out to sites (where it is administered)
Johnson & Johnson / Jannsen vaccine
• Announcement earlier this week that Medsafe (regulatory authority) granted provisional
approval of the Janssen COVID-19 vaccine for individuals 18 years of age and older. The
medical evidence shows Janssen is a very safe and effective vaccine.
• NZ secured 2 mil ion doses of the Janssen vaccine through an advance purchase agreement
last year. We purchased a portfolio of vaccine options to provide us with flexibility, great to
have approval now confirmed.
1982
• The Janssen COVID-19 vaccine has also received emergency or provisional approval in
Canada, USA and Australia.
• Plan remains to ramp up the rol -out using the Pfizer vaccine from here, having the option of
Act
the Janssen vaccine increases our choices and provides us with flexibility if we need it. As a
single dose vaccine, it may be useful in hard to reach locations or emergencies, or for those
who cannot get the Pfizer vaccine.
• Provisional approval is the first step in the process. Cabinet wil weigh up the options on the
best use of the Janssen vaccine fol owing advice from officials. A Cabinet ‘decision to use’
can be expected sometime in August.
• It’s good to have a range of options to access safe and effective vaccines to meet the need of
New Zealanders now and in the future.
Clinical
Information
• Medsafe fol ows a rigorous assessment process informed by the most up to date medical
and scientific data. Approval has been very carefully considered with safety the key priority.
• Programme includes a clinical quality and safety team incl external expertise.
• Programme includes post event monitoring team; works with Centre for Adverse Event
Monitoring (CARM)
• Pfizer vaccine We have secured 10 million doses – enough for 5 mil ion people to get the 2
Official
doses they need to be protected. It works by teaching your immune system to recognise and
fight off the virus. Second dose of the vaccine
at least 21 days (3 weeks) after your first
dose.
the
• The Pfizer vaccine:
• is a messenger RNA (mRNA) vaccine
• does not contain any live virus, or dead or deactivated virus
• can't give you COVID-19
• can't affect your DNA
• does not contain any animal products.
under
How effective is the COVID-19 vaccine, and what does 95% mean?
As with any vaccine, the Pfizer vaccine (Comirnaty) may not fully protect everyone who gets it.
However, it is highly effective if people have both doses. That means, if you do catch COVID-19,
you’re far less likely to fall seriously ill and less likely to transmit the virus to others. Studies have
shown that about 95% of people who receive both doses were protected against getting seriously ill.
The COVID-19 vaccine stimulates your body’s immune system to produce antibodies and other
Released
proteins that wil fight the virus if you’re exposed to it. This reduces the risk of getting infected and if
you do get COVID-19, it means you could have no symptoms or will have much fewer, milder
symptoms and recover faster.
3
COVID-19 messages for Business NZ meeting - 9 July 2021
While the data is clear that vaccines protect people from the effects of COVID-19, research is
ongoing to determine whether a vaccinated person could still transmit the virus to someone else –
so to be safe, we must assume there is still a risk of transmission.
The difference between efficacy and effectiveness
Efficacy is the measure used in clinical trials. Efficacy measures how well a vaccine can prevent
symptomatic infection (and sometimes transmission) in clinical trials. This is under ideal and
1982
controlled conditions, comparing people who receive the vaccine with those who receive a saline
placebo.
Act
Effectiveness is the measure used in the real-world. It is how wel the vaccine performs in the real
world outside of the clinical trials in a mixed population. We would expect a vaccine with a high
efficacy to be highly effective in the real-world, but these measures are unlikely to be the same.
The efficacy of the Pfizer vaccine (Comirnaty) was measured in two ways.
Phase 1 clinical trial – level of antibodies. The immune response to the vaccine was measured by
looking at the level of antibodies in the bloodstream and how well they worked to neutralise the
COVID-19 virus in laboratory tests.
Phase two and three clinical trials – vaccine and placebo. The efficacy of the Pfizer vaccine was
tested in about 44,000 participants aged 16 years and over where COVID-19 was already circulating
Information
in communities. About half of these participants were randomised to receive the vaccine and the
other half received a saline placebo. The trial looked at how many people got COVID-19 symptoms
after they were vaccinated compared to how many got COVID-19 after getting the
placebo. Participants had two doses of the vaccine or placebo, getting their second dose within 19 to
42 days after their first dose. They were then closely monitored and evaluated for at least 2 months
after their second dose.
Official
A consistently high efficacy of over 92% was observed in the clinical trials across age, sex, race,
ethnicity and people with underlying medical conditions. This means after getting the Pfizer vaccine,
the
more than 9 out of 10 people are protected against COVID-19 regardless of their age, health status
or ethnic group.
Long-term efficacy - to understand the long-term efficacy and safety of the vaccine, participants in
the clinical trials are being tracked for another two years after their second dose of the Pfizer
vaccine.
under
Getting your second dose increases protection. For the best protection, impt to get second dose at
least 3 weeks after first dose. Clinical trials showed the Pfizer vaccine (Comirnaty) had a higher
efficacy against symptomatic COVID-19 infection after receiving the second dose. This is supported
by recent real-world data.
The first dose ‘primes’ your immune system but protection doesn’t last as long because the level of
antibodies fal s. A second dose gives your immune response a boost – with lots more antibodies to
Released
help your immune response to mature and provide longer protection.
In the COVID-19 vaccine clinical trials, people were followed very closely for side (adverse) effects for
2 months after the second dose of the vaccine. They compared the results between people who had
and hadn’t been vaccinated. To understand the vaccine's long-term effectiveness, safety, and side
effects, participants in the clinical trials are tracked for another two years. This is from their second
4
COVID-19 messages for Business NZ meeting - 9 July 2021
dose of the Pfizer vaccine. Participants will have their health monitored and attend regular fol ow-up
visits. This clinical trial data is closely monitored by Pfizer/BioNTech and an independent group of
experts called the Data Monitoring Committee.
Side effects -like all medicines, the vaccine may cause side effects in some people. Most side effects
are mild and don’t last long — they're more common after the second dose. They won’t stop you
from having the second dose or going about your daily life. Some side effects may temporarily affect
your ability to drive or use machinery. In the clinical trials, common side effects were reported in
every 1 in 10 to 1 in 100 people. These include:
1982
• pain or swelling at the injection site
• feeling tired or fatigued
Act
• headache
• muscle aches
• chills
• joint pain
• fever
• redness at the injection site
• nausea
Uncommon side effects - In the clinical trials, uncommon side effects were reported in every 1 in 100
to 1 in 1,000 people. These include:
Information
• enlarged lymph nodes
• feeling unwel
• pain in limb
• insomnia
• itching at injection site
Official
Rare side effects - In the clinical trials, temporary one-sided facial drooping was reported in every 1
in 1,000 to 1 in 10,000 people. the
Allergic reactions - Serious allergic reactions do happen but are extremely rare. They usually show
soon after you’ve had your vaccine, which is why you need to wait at least 20 minutes.
International travel
under
Vaccine passports – working with other jurisdictions on this. Requires consistent global effort
EVA – early vaccine access process for certain criteria
If you need to travel overseas from New
Zealand, you can apply for an early COVID-19 vaccine on compassionate grounds or for reasons of
national significance. You can now apply for early access to the vaccine if you must travel overseas
from New Zealand on or before 31 August 2021. You'l need to apply at least four weeks before you
travel. Application process for early access to the vaccine is different depending on your reason for
overseas travel.
Released
compassionate grounds
• access critical medical care that is not available in New Zealand for yourself or yo
ur dependant
• visit
an immediate family member who is dying
• provide critical care and protection for a
dependant eg, your child.
5
COVID-19 messages for Business NZ meeting - 9 July 2021
reasons of national significance
•
to protect the safety and security of New Zealand’s right to govern itself
•
for Government-approved humanitarian efforts as part of New Zealand’s commitments to foreign
aid, international disaster responses, or supporting Pacific and Realm countries' recovery from the
COVID-19 pandemic
•
to participate in major international events where travel is necessary to represent New Zealand
•
for national y significant trade negotiations.
Agency sponsors wil apply on your behalf. The agency sponsor responsible for the international
event must apply on behalf of those travelling for reasons of national significance. We can't accept
applications from individuals. Agencies will contact individuals that are part of a group they're
making applications for.
Overseas travel that won't be considered
•
to a country New Zealand has approved for quarantine free travel, e.g. Australia and the Cook
Islands
•
for private, recreational or commercial travel
•
to reunite with family
•
to attend a funeral or memorial service
•
to attend a school or university.
Legacy
Very likely to require booster vaccination programme, turning our minds to that
Legacy systems – new workforce; CIR, booking system, new ways of working e.g. workplace
vaccinations contracted with MoH or DHBs.
All information:
https://www.health.govt.nz/our-work/diseases-and-conditions/covid-19-novel-
coronavirus/covid-19-vaccines/
https://www.health.govt.nz/our-work/diseases-and-conditions/covid-19-novel-
coronavirus/covid-19-vaccines/covid-19-vaccine-strategy-planning-insights/covid-19-supporting-
vaccine-rol out#workplace
under the Official Information Act 1982
Released
6

 Document 2
Document 2
1982
BioNTech/Pfizer COVID-19 Act
Vaccine and Immunisation
Programme
Information
Planning blueprint:
Official
Workplace sites
the
V
ersion 9, Issue 2
09 July 2021
under
Released
link to page 12 link to page 12 link to page 14 link to page 17 link to page 25 link to page 28 link to page 28
Contents
1.
Purpose .................................................................................................... 3
2.
Service model .......................................................................................... 3
3.
Early planning considerations .................................................................. 5
4.
Information for Vaccination providers ..................................................... 8
5.
Information for Workplaces/Employers ................................................ 16
Appendix 1: Guidance on delivery models for Māori, Pacific, ethnic
1982
communities and disability groups .................................................................. 19
Act
Information
Official
the
under
Released
2
1. Purpose
This document provides guidance to commissioning agencies (the Ministry of Health and District
Health Boards), vaccination providers and workplaces/employers in their planning for vaccine
delivery to Group 4 in workplaces. It is designed to help workplaces/employers and vaccination
providers decide if they can participate in this programme, and if so what planning considerations
do they need to consider before rol ing out on-site vaccination.
1982
2. Service model
Act
The COVID-19 Vaccine and Immunisation Programme (CVIP) has the following success goals to
achieve balanced decision making:
• Honours and upholds Te Tiriti o Waitangi principles.
• Quality and safety – vaccines and immunisation processes are clinically and culturally safe,
backed by a strong evidence base, appropriate kaupapa and capability.
• Experience – renewed/increased trust and confidence in the health sector and
immunisation, underpinned by positive experiences at system, programme and
Information
whānau/individual levels.
• Equity – Māori, Pacific and people with disabilities achieve equitable immunisation
outcomes. Everyone in New Zealand and the Pacific has equal opportunity to access the
vaccine.
• Access – New Zealand’s and Pacific’s immunisation needs are met at the right time and
Official
place with minimal waste.
Offering different service models is one way the programme can work towards these success
goals. The four service delivery models for the CVIP are:
the
• community sites (in existing healthcare facilities e.g. general practice, community
pharmacy, Hauora practices, urgent care)
• hospital sites
under
• temporary sites (e.g. workplace, marae, church)
• fixed sites (e.g. community hubs).
Workplaces are ‘temporary sites’ designed to enable employers and vaccination providers to
leverage their experience and resources to vaccinate workers at a time and place convenient to
them. This model is considered important to drive uptake, by making access convenient and easy
(such as in rural communities) and supports equity of access for Māori and Pasifika populations.
It is considered an effective model, as demonstrated by the annual influenza campaign and the
Released
rol out of the Pfizer vaccine by occupational health vaccination providers to workplaces in Groups
1 and 2.
3
The Ministry of Business, Innovation and Employment has published guidance for employers on
supporting the vaccination campaign at
https://www.employment.govt.nz/leave-and-
holidays/other-types-of-leave/coronavirus-workplace/covid-19-vaccination-and-employment/
The Public Service Commission has issued guidance for public sector agencies, which includes the
line “Vaccines should be administered in the workplace where possible”.
https://www.publicservice.govt.nz/resources/covid-19-workforce-vaccinations-guidance/
This model is for delivery as part of the Group 4 rollout that will start from late July 2021.
2.1 Eligibility criteria for workplaces/employers
1982
Workplaces must:
Act
Either;
• be considered one of the largest workplaces/employers (by size of workforce) and have
enough workers per site to be vaccinated. As a guide, an indication is workplaces with 1,000+
employees, with the ability to vaccinate several hundred staff per site, or
• for smaller workplaces/employers – support a DHBs’ equity goals of targeting workplaces
with high Māori, Pacific or ethnic populations and those harder to reach (e.g. due to rurality
or shift work). Note even for smaller workplaces/employers – there is a minimum
requirement of 70 vaccinations per site per day
And;
Information
• have had a successful vaccine programme previously delivered onsite
• be able to provide staff to undertake specified roles and responsibilities as outlined in the
Workplace Model Planning Blueprint – particularly the logistical tasks to support worker
engagement, recall processes, and cultural and religious safety.
Official
2.2 Families/Visitors/Neighbouring Businesses to Sites
the
It is recommended that family and visitors are not part of the workplace/employer site
vaccinations to prevent any public/crowd control issues, ensure compliance with the Health and
Safety at Work Act is maintained, and Public Liability risks are managed. As the employer and
provider gain experience with the Pfizer vaccination processes, in conjunction with the
under
vaccination providers commissioning agency, they may reconsider this position.
Released
4
3. Early planning considerations
There are some notable differences between the Pfizer vaccine and other vaccine programmes,
such as influenza, that both workplaces/employers and vaccination providers should be aware
of. The fol owing considerations have been shared by occupational health vaccination providers
who delivered the Pfizer vaccine in Group 2 eligible workplaces:
• The Pfizer vaccine is a national roll out with the goal of offering vaccination all peoples in
Aotearoa New Zealand within a set timeframe.
1982
• It requires the administration of two doses separated by at least 21 days. This requires
the onsite delivery to be replicated twice.
Act
• It is a delicate vaccine:
o Nationally, it is stored at ultra-low temperatures and cannot be refrozen once
thawed.
o It is transported to vaccination providers at +2C to +8C and has 31 days of expiry
at this temperature.
• It has different logistical constraints:
o It is provided in boxes of five, 15, 195 vials. Information
o Each vial contains multiple doses (six or seven) and sites must be able to
administer at least 30 doses per day if moving in mobile chilly bins and if cold chain
is maintained.
• Due to the price per dose and other associated costs, there needs to be a minimum
number of doses delivered in a sitting to be viable for vaccination providers, this is
approximately 70.
Official
• There are additional administration and information requirements;
o Vaccination providers need to be prepared to answer more questions on the
the
vaccine than they experience in other vaccine programmes.
o Recording every vaccination in the COVID-19 Immunisation Register (CIR) is
mandatory and must be done on the same day as the vaccination.
• There are additional workforce requirements:
under
o A three-person team’s minimum needed at a site (two clinicians, one
administration) instead of one nurse for Influenza.
• There are additional physical location considerations;
o Workplace staff need to stay in active observation for at least 20 minutes post
vaccination event – there needs to be adequate space to al ow for this.
o There needs to be dedicated private and appropriate space available for the
Released drawing up of doses to ensure vaccination providers can concentrate on this
process given there are multiple doses to draw per individual vial, and maintain
adequate IPC protocols.
5
o Privacy for workplace staff as they wil be answering people’s questions and
recording details in the CIR.
o Suitable area for stage two observation, as required (including access to
stretcher/bed and privacy screening). (refer Programme Standards).
• There are additional vaccine transport considerations;
o Vaccination providers may need to assess the suitability of existing chil y bins and
ensure they meet the standards for their use.
1982
o Prepared doses cannot be transported to other sites.
3.1 How many people can be catered for?
It is expected that vaccination providers and workplaces/employers work together to determine
Act
the best throughput plan for each site. As each workplace is likely to have different needs and
variables, the fol owing information is to support joint planning and decision making related to
individual site throughput.
•
Financial minimum viable product (MVP)
Based on initial assessment of the financial MVP, no less than 70 vaccinations can be
administered in a session (day). Variables will change depending on individual vaccination
provider and workplace/employer constraints. A tool has been developed to assist
vaccination providers calculate the MVP for each situation.
Information
•
Logistical constraints
If the number of workers vaccinated per session, for whatever reason, drops below the
financial MVP then the fol owing applies:
o Based on pack size, a minimum of 30 vaccinations, per vaccine delivery, will need
Official
to be administered, as the minimum delivery is five vials containing six doses.
o To mitigate possible vaccine delivery delays, it is recommended the 30
vaccinations are planned to be completed within 1 day.
the
•
Infection, prevention and control (IPC) requirements
IPC requirements are critical considerations for planning.
Once the vaccine has been diluted, it must be administered within six hours. Any
prepared doses not used within this time period must be discarded.
under
It is recommended vaccination providers are vigilant of vaccine expiry times and factor in
time for date-stamping.
•
Physical site
The number and size of available rooms onsite.
Physical capacity of waiting rooms to meet the 20-minute minimum of the post-
vaccination observation period should be considered when estimating throughput.
Released
•
Vaccination provider workforce
The number of vaccinators available through the provider, trained first aid and support
staff, in relation to the number of people being vaccinated.
6
The current average number of doses delivered per vaccinator per hour is 12.
•
Workplace/employer operating hours
The business hours the workplace/employer deem appropriate and the flexibility of the
vaccination provider.
The workplace/employer’s workers working hours will determine available numbers of
workers per session. For example, there could be 500 workers, but they work shifts and 1982
therefore only a portion may be available during the same period. Shift workers who
cannot be interrupted need to be taken into account.
Workplaces/employers may need to give vaccination providers with access to workplaces
Act
after hours.
Further planning to manage people returning for their second dose and new people, such
as new workers, getting their first dose.
Information
Official
the
under
Released
7
4. Information for vaccination providers
Vaccination providers must submit a delivery plan to their commissioning agency as part of pre-
contracting due diligence. Content in this document will assist in the planning required to
develop that plan.
Planning for this model should be done in conjunction with the Programme Standards and
Operating Guidelines.
The document map below outlines where this document sits in relation to the wider suite of 1982
resources.
Act
Information
Official
the
4.1 Commissioning of vaccination providers
under
A vaccination provider wil either be commissioned by the Ministry of Health (MoH) or a District
Health Board (DHB).
•
MOH will only commission existing occupational health vaccination providers who can
serve workplaces/employers that are geographically spread across multiple DHB
boundaries.
•
DHBs wil utilise any workforce/provider to best serve workplaces/employers residing
in their geographical boundary.
Released
MoH and DHBs wil operate an ‘open book’ process to col aborate on what vaccination providers
are being commissioned to serve what workplaces. They are responsible for designing,
communicating and implementing how vaccination providers will be commissioned and how
workplaces/employers can engage in the programme. They are also responsible for the on-
boarding of new providers into the programme.
8
Both commissioning agencies should consider equity, accessibility and acceptability when
commissioning vaccination providers.
4.2 Eligibility criteria for vaccination providers;
Vaccination providers must:
• be established vaccination providers with a Health Provider Index number
• have experience in delivering a vaccine programme
• have a workforce that can ensure cultural and religious safety of workplace staff; they must 1982
have clearly defined clinical quality and equity leads.
• be able to comply with the Programme Standards, Service Specifications, Immunisation
Handbook and Operating Guidelines including:
Act
• existing cold chain accreditation
• active clinical quality and safety oversight mechanisms
• mandatory use of the National Booking System and cal centre
• Deliver to the set Price per Dose
• be able to operate with agility, as the programme evolves, and be prepared to engage in the
development of the programme.
4.3 Providing a safe and quality vaccination experience
No matter where a COVID-19 vaccination service is located, mana manaaki must underpin the
Information
vaccination experience for everyone in Aotearoa New Zealand. This includes a culturally and
clinically safe vaccination experience.
A ‘one size fits all’ approach to service delivery will not work for our priority population groups.
Different considerations wil be required dependent on a consumer’s health and/or disability,
where they live, and how they access services. Refer to Appendix 1 for guidance on delivery
Official
models for Māori, Pasifika, ethnic communities and disability groups.
4.4 Clinical quality management systems and governance
the
Clinical safety and quality requirements are sourced from the MoH Immunisation Handbook,
Operating Guidelines, and Programme Service Standards. It is expected that vaccination
providers delivering the workplace model have active clinical governance and systems in place,
including a defined quality and safety lead. In addition, each DHB region must have appropriate
quality and safety oversight of the vaccination programme rol out through their existing quality
under
and safety and/or clinical governance mechanisms. Vaccination providers contracted directly to
the Ministry must also have clearly documented clinical quality and safety assurance people and
processes (as per the documents above – Immunisation Handbook, Operating Guidelines, Service
Standards), including reporting mechanisms for review of significant events and accountable
clinical leads and quality leads.
Vaccination providers must submit a delivery plan to their commissioning agency that includes;
• an overview of existing clinical quality and safety systems; at a minimum, this includes
Released
oversight of adverse events, complaints, risk and incident management (note: in this
context, ‘adverse event’ does not refer to an adverse reaction fol owing vaccination)
9
• names and contact details for clinical lead(s) and quality manager(s), and details of their
clinical governance/quality and safety groups within their organisation including
frequency of meetings and responsibilities.
4.5 Business Continuity Planning
Business Continuity Plans should be in place to; manage disruption to systems (CIR, Booking,
Internet access etc), manage impact to business as usual in the event of surge demand and
manage impact in the event of COVID-19 alert level changes or vaccine loss or wastage. Refer to 1982
the Operating Guidelines for additional guidance.
In the event of COVID-19 alert level changes, vaccination providers will follow all government
Act
direction regarding continuation or not of the Pfizer vaccine programme.
4.6 Vaccination workforce
Vaccination workforce decisions should be considered in alignment with the Operating
Guidelines, the Immunisation Handbook and clinical resources provided by the Immunisation
Advisory Centre (IMAC).
4.7 Vaccination Training
MoH has partnered with IMAC to provide the mandatory training required to administer the
COVID-19 vaccine. This includes clinical training for the Pfizer vaccine and non-clinical training for
Information
using CIR. Evidence of completion of mandatory training is required, as well as any training
undertaken to be an authorised vaccinator.
Commissioning agencies may seek to supplement this training by providing practical observation
of operating vaccination centres if appropriate.
4.8 Planning for booking appointments Official
Workplaces/employers and vaccination providers wil need to col aborate and be flexible with
regards to appointment timings and what is feasible for both parties at any given site. This is
the
especial y the case with workplaces with shifts and where it is difficult to take workers out of
work.
4.9 Engagement and invitation
The workforce model has a fixed population group to serve. Workplaces/employers are
responsible for engagement and communication with their workers. Vaccination providers are
under
responsible for communicating when and where they wil be onsite and when there are any
changes to planned bookings/sessions.
4.10 Booking appointments
Vaccination providers are responsible for managing appointment bookings. It is mandatory for
providers to use the CVIP National Booking System.
MoH recommends al sites open bookings at least one week before vaccinations start to al ow the
Released
provider to supply an accurate order of vaccine and consumables, and ensure adequate time to
communicate with workers at the workplace. Having a clear idea of the number of bookings wil
also al ow vaccination providers to staff sites to meet demand.
10
However, it is highly likely that workplaces will need to open bookings well in advance of one
week, depending on individual business planning needs. Workplaces/employers and
vaccination providers will need to collaborate and be flexible with regards to appointment
timings. This is especial y the case with workplaces with shifts and where it is difficult to take
workers out of work.
MoH recommends second appointments are booked with vaccination providers while
individuals are onsite for their first dose.
4.11 Worker follow up
1982
If a worker either:
• did not respond to the initial invite
Act
• did not attend the first dose booked appointment
• do not have a second dose booked
• did not attend the second dose booked appointment.
As the vaccination provider runs the booking process/system, they are responsible for worker
fol ow up with the direct support of the workplace/employer. Where this requires the sharing of
individual data to an employer, such as who has had a vaccination and who has not, consent must
have been obtained from employees prior.
Information
4.12 Second dose follow up
Vaccination providers should consider how demand planning wil cater for individuals who only
receive a single dose at the workplace vaccination site – for example, they choose to get their
second dose at a different location or got their first dose elsewhere.
4.13 Administering leftover vaccines Official
Wastage through leftover vaccine should be actively minimised by planning a back-up or
standby list. Vaccination providers are responsible for administering vaccine before expiry. Any
wastage must be reported in the CIR and mitigated for future vaccination events.
the
Workplaces/employers are responsible for managing a process to invite non-booked workers to
utilise leftover vaccines.
4.14 Onsite functions
The table below outlines the required onsite functions and responsible parties.
under
Depending on the operating hours and size of the site, the number of people fil ing roles in these
function areas may vary; however, the functions across sites won’t change.
Functions on site
Responsible / accountable for
Site operations
Responsible for onsite inventory management.
Released
Should any assistance be required, provide or access
another basic life support trained adult onsite to manage
and/or deliver the appropriate response.
11
Clinical oversight
Nominated COVID-19 clinical on-site lead who will
coordinate all vaccination activities including vaccine
logistics (ordering, receiving and storage). (Note: this is not
the same role as the over-arching clinical lead with a local
governance role, as per the Programme Standards).
Must have vaccination experience in order to be responsible
for al clinical aspects of the vaccination site which can
include:
1982
• providing on-site clinical advice and guidance
including managing any adverse events following
vaccine (AEFI)
Act
• ensuring that equipment and medications for the
management of medical emergencies, including
anaphylaxis, is available, consistent with the
programme Service Standards, and considers
specifics of the site (i.e. remoteness)
• running a closed ‘dry run’ session with provider staff
• leading team huddles pre- and post-vaccination
clinics
Information
• submitting significant event analysis reporting to the
relevant DHB and/or the Centre for Adverse
Reaction Monitoring (CARM) as necessary.
Welcoming (including
Confirm the NHI number and workplace staff details.
Official
registration)
Provides information to gain informed consent.
Checks that workplace staff are well.
the
Vaccination preparation
Dilutes and draws up vaccine in line with established IPC
protocols and vaccine preparation guidance.
Second person checks the processes and vaccine dose and
confirms vaccine vial information.
under
Vaccination administration
Confirm identity (does not require being shown an
identifying document).
Ensures workplace staff are ready for vaccination, aware of
potential side effects, conducts the pre-vaccination clinical
assessment, etc.
Gains informed consent.
Released
Administers vaccine.
12
link to page 22
Post vaccination monitoring
Observe individuals post vaccination to monitor for possible
adverse event. Should any assistance be required, this
function wil manage and/or deliver the appropriate
response and liaise with emergency providers as
appropriate.
4.15 Adverse events
The provider-nominated site clinical lead is responsible for the clinical management of vaccine 1982
related adverse events at the place of vaccination. Vaccine-related adverse events must be
recorded and reported to CARM and to the workplace/employer lead. These can be reported
through:
Act
• the CIR for adverse events during the observation period.
• the CA
RM website1 for any post event presentation after the workplace staff member
has left the site.
Further functionality on CARM reporting and other systems for ease of access is being explored.
Refer to the Programme Standards for information on emergency equipment required on site.
4.16 Vaccination provider workforce capacity
Vaccination providers should consider how to manage their BAU activities while delivering COVID
Information
vaccinations, including how sustainable it is to deliver BAU activities alongside vaccinations.
4.17 Vaccine and consumables logistics
The Operating Guidelines have specific information on vaccine supply chain and onsite storage
requirements for the Pfizer vaccine, including that fridges must have the ability to detect
temperature breaches.
Official
Commissioning agencies and their respective vaccination providers must agree a delivery plan of
the vaccine and consumable stock reflective of the anticipated throughput.
the
The first initial vaccination sessions at a new site should be at a reduced scale to test systems and
processes before scaling vaccine administrations later.
It is essential that supply is planned for within a 31-day expiry time. Delivery planning must factor
in the fol owing logistics constraints:
under
• Minimum six people must be vaccinated within six hours.
• Minimum 30 vaccinations need to occur within 1 day.
Stock deliveries can be made seven days a week. Stock is delivered in packs of five vials (30
doses), 15 vials (90 doses) or a tray (1170 doses) at standard 2-8˚C cold chain.
Stock must be ordered two days in advance to ensure provision for the variances in demand that
can occur daily.
Released
An identified person at each site must manage vaccine and consumable stock. This wil al ow for
effective management of vaccine and consumables with the ability to order new stock.
1 https://report.vaccine.covid19.govt.nz/s/
13
4.18 Onsite IT requirements and support
Every vaccination given must be recorded in the CIR.
The supporting equipment and infrastructure to access the CIR is outlined in the Operating
Guidelines and includes access to high-speed internet, a laptop, computer or tablet, and a
separate smartphone.
Currently, each person accessing the CIR requires a non-public email address. This is required to
mitigate security concerns on access to the CIR and supporting information.
Vaccination providers should ensure there is an available Superuser (someone who is a frequent 1982
and competent system user) to provide local support.
4.19 Consumables
Act
Consumables listed in the Operating Guidelines will be provided directly to sites from the
distribution provider. Other consumables not specified in the Operating Guidelines should be
covered by vaccination providers within the provided funding.
In the event of COVID-19 alert level changes, PPE is to be sourced through existing channels.
4.20 Other equipment requirements
Minimum equipment standards for management of medical emergencies are outlined in the
Programme Standards document. Additional equipment provided for use at the site must take
into consideration the accessibility of the site to emergency services, remoteness, and the skil
Information
sets of on-site vaccination providers.
4.21 Physical locations
Vaccination providers must ensure the workplace/employer site is appropriate for use according
to the Programme Standards and Operating Guidelines.
Official
When choosing the physical site for vaccinations at the workplace, consideration needs to be
taken about whether the physical space available wil support the volume planned for the site
and the end-to-end administration process.
the
How the site is arranged, and the throughput, wil depend on a range of factors, including the
size of the site.
Space must be available for people to remain on site for at least 20 minutes after their vaccination
so they can be observed. Space and appropriate equipment must be available for stage two
under
recovery observation, as outlined in the Programme standards.
Emergency vehicle access must be identified in case of an adverse event.
There must be consideration of how a space may be rearranged or throughput reduced in the
event of COVID-19 alert level changes. The site must be set up so it is easy to see most areas
used for the immunisation process and provider staff must be able to communicate easily if they
need help.
4.22 Site readiness self-assessment check list
Released
Vaccination providers must complete the ‘site readiness checklist’ and submit to their respective
commissioning agency.
14
4.23 Funding and reporting
Vaccination providers cannot charge workplaces/employers for any costs associated with the
delivery of the Pfizer vaccine.
The MoH set Price per Dose (PPD) (both for during and after business hours) for varying providers
and reporting requirements are available in the relevant service specifications.
The PPD for Occupational Health Providers is
$33.91 for ordinary hours, and
$46.59 for after
hours. Both prices are inclusive of recalls.
"Out of hours" is defined as:
1982
(a) 8pm to 8am the next day, Monday to Thursday; or
(b) 5pm Friday to 8am Monday; or
Act
(c) any Public Holiday.
Where a DHB commissions a primary care provider, the approved PPD for primary care will be
applied.
From 1 July 2021, an automated PPD payment solution wil be implemented based on the events
recorded in CIR. When an immunisation event is appropriately recorded in CIR, the combination
of contract, provider and site wil be used to automatical y determine the payment amount due
and the contracted party will receive payment for the service automatically. It will be not be
necessary to generate and send additional invoices for PPD services.
Information
Where a contract is not on a PPD basis, or where there are special payment arrangements outside
of the national PPD pricing arrangement, these will usually be managed on an invoice basis.
Full terms and conditions for payment of COVID-19 vaccinations services will be specified in
individual contracts with the commissioning agency.
Official
the
under
Released
15
5. Information for Workplaces/Employers
5.1 Supporting providers
Workplaces/employers need to work alongside the vaccination provider serving their workers.
This includes inputting into and agreeing the delivery plan vaccination providers must provide
their commissioning agency, which wil include the volume of workers to be vaccinated and the
booking schedule and approach.
It will be critical for workplaces/employers to consider physical locations that vaccination 1982
providers wil need. This includes consideration about whether the physical space available will
support the volume planned for the site, including the space for the minimum for the 20-minute
active observation area, emergency vehicle access, and consideration of how a space may be
Act
rearranged or throughput reduced in the event of COVID-19 alert level changes.
Workplaces/employers also need to provide onsite support, such as access to high-speed
internet and
support during the vaccination sessions as laid out below.
5.2 Planning for booking appointments
Workplaces/employers and vaccination providers wil need to col aborate and be flexible with
regards to appointment timings and what is feasible for both parties at any given site. This is
especial y the case with workplaces with shifts and where it is difficult to take workers out of
work.
Information
5.3 Engagement and invitation
The workforce model has a fixed population group to serve. Workplaces/employers wil need to
communicate to workers through existing employer channels to promote the opportunity,
provide information about who the vaccination provider is and when/where they wil be onsite
and how to book.
Official
Workplaces/employers are strongly encouraged to work directly with Māori, Pacific and ethnic
staff in their workplace to ensure communication and engagement is tailored appropriately.
the
5.4 Booking appointments
Vaccination providers will manage booking systems. It is mandatory for all providers to use the
CVIP National Booking System. Workplaces/employers wil need to support workers who need
assistance with making a booking.
under
MoH recommends second appointments are booked with vaccination providers while
individuals are onsite for their first dose.
5.5 Worker follow up
If a worker(s) either;
• did not respond to the initial invite
• did not attend the first dose booked appointment
Released
• do not have a second dose booked
• did not attend the second dose booked appointment.
16
Workplaces/employers wil need to provide support to the vaccination provider to provide fol ow
up. Where this requires the sharing of individual data such as who has had a vaccination and
who has not, consent must have been obtained from the employee prior for this data to be
shared with their employer.
5.6 Administering leftover vaccines
Wastage through leftover vaccine should be actively minimised by planning a back-up or
standby list. Where vaccine is available due to booked staff not turning up, the
workplace/employer needs to have a process in place to invite other workers on site to take up 1982
the opportunity.
5.7 Onsite functions
The table below outlines the required onsite functions that workplaces/employers are
Act
responsible for:
Functions on site
Responsible / accountable for
Traffic and site management
Manage traffic and people flow in and out of the carpark
(non-clinical)
and venue (if required).
Oversight and management (including health and safety) of
site. Ensure facilities meet the Programme Standards by
Information
way of adequate facilities, such as bathroom access.
Hauora support
Across the vaccination pathway (from welcoming to post-
vaccination monitoring), provide support for the wel being
of the people seeking a vaccine.
Official
Welcoming (including
Meet people and manage flows of people to keep social
registration)
distancing regardless of COVID-19 alert level.
Identify
the whether there are additional supports or
considerations required to facilitate an inclusive, safe and
accessible experience for workers. Refer Appendix 1.
Ask if second dose appointment has been made in advance,
encourage staff to book for this if not.
under
Post vaccination monitoring
Sign off workers as fit for work and able to return to work,
or sent them home if unwel .
5.8 Adverse events
Released
If a vaccine related adverse event occurs on-site while the worker is under the care of the
vaccination provider, that provider is responsible for the clinical care and reporting of the event.
Refer to vaccination provider section for more information.
17
Vaccine-related adverse events must be reported to a nominated workplace/employer lead in
the event a notification to WorkSafe is required.
Where an employee experiences a vaccine related adverse event after they have completed the
required 20 minute observation period, and the provider is no longer on site, the employee or
employer can call Healthline on
0800 358 5453, or if they are concerned about their safety, cal
111. Tel them they’ve had a COVID-19 vaccination so they can assess them properly. More
information about adverse events and how to report them, is available on the MoH Website
https://www.health.govt.nz/our-work/diseases-and-conditions/covid-19-novel-
coronavirus/covid-19-vaccines/covid-19-vaccine-side-effects-and-reactions.
1982
Liability for the CVIP is consistent with other onsite vaccination programmes, such as influenza.
Act
Information
Official
the
under
Released
18

Appendix 1: Guidance on delivery models for Māori,
Pacific, ethnic communities and disability groups
The fol owing examples show how an equitable approach to Māori, Pacific, ethnic and disabled
communities may be incorporated into vaccination site planning and operations. Note that these
are examples of equitable practices to support your planning, not a checklist of requirements
that you must meet.
1982
Act
Information
Official
the
under
Released
19
link to page 29
1982
Act
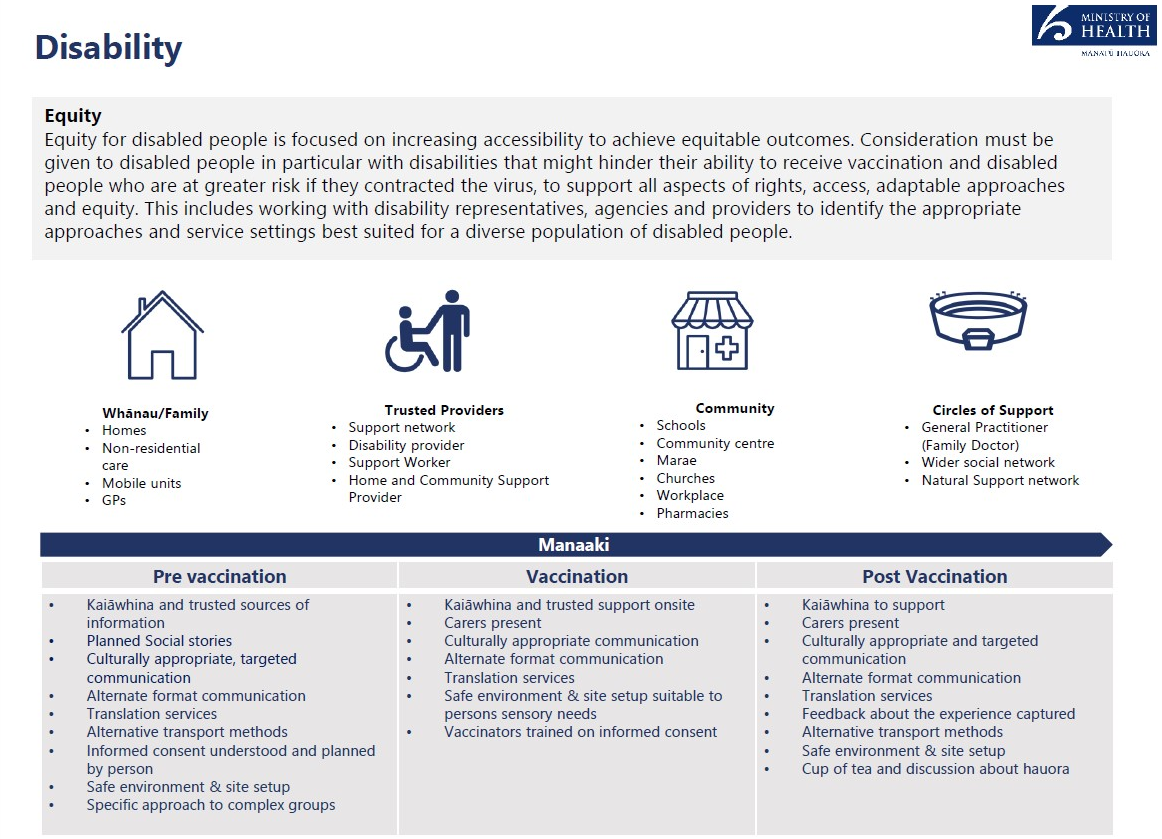
In order to deliver an equitable vaccination programme, vaccination services and settings
Information
must be inclusive and accessible vaccination options for disabled people and their
communities. This includes consideration of accessibility across the vaccination journey, for
example, providing early information on the benefits of vaccination and awareness of service
delivery options and associated accessibility features.
Al vaccination sites (with the exception of mobile sites delivering to specific groups) must
meet accessibility standards.
2
Official
Core components include
• Appropriate disability specific acco
the mmodations in all sites.
• Alternate formats translations of all public facing communications and engagement.
• Developed supported decision-making process in alignment to of the Health and
Disability Code Of Rights (Right 7).
under
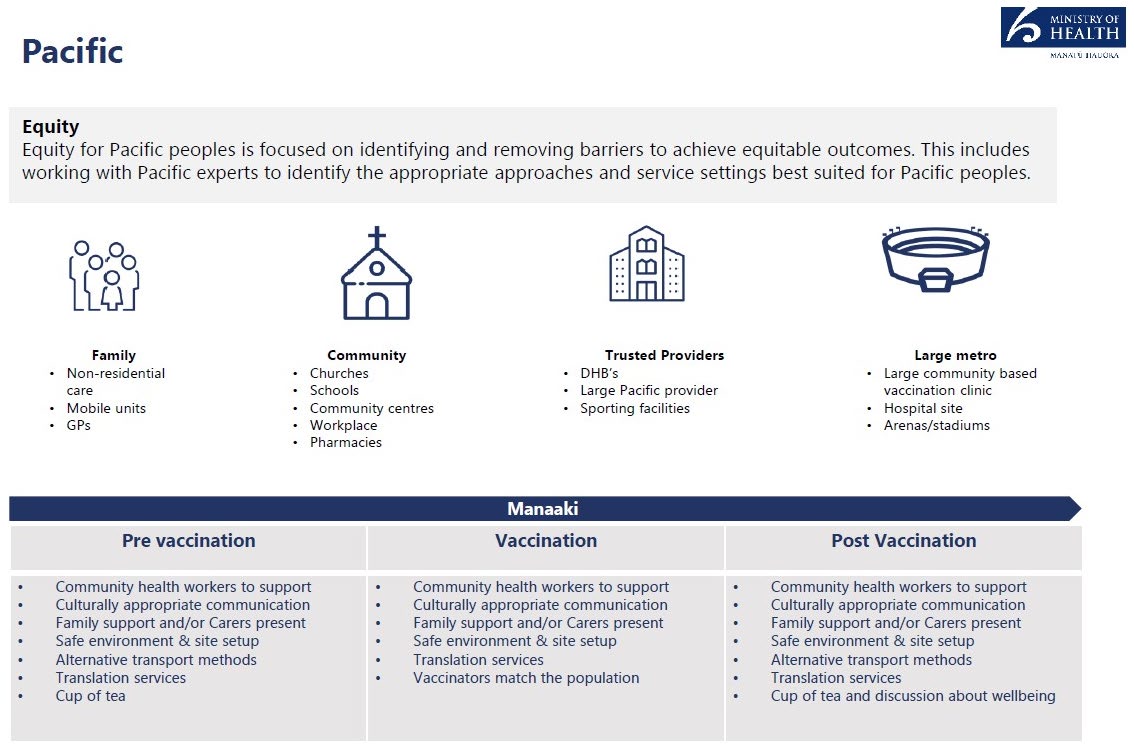
Ethnic Communities
Equity for ethnic communities can be achieved by targeting members of the community who
cannot ordinarily be reached because of communications barriers and lack of understanding of
the health system. The target approach involves enagaging with community leaders and
stakeholders, including faith and religious leaders, local champions, and engagement teams who
can encourage and relay key information on the vacine rollout.
Released
2 NZS 4121:2001 Design for access and mobility: Buildings and associated facilities.
Ministry of Health
guidance
20
Family
Community
Trusted vaccination Large Metro
providers
GPs
Churches, Mosques, DHBs
Community base
Mobile sites in local Temples and
Large ethnic
event vaccination
communities
Gurdwara
vaccination
clinic
Community centres
providers
Hospital sites
Workplaces
1982
Schools
Pharmacies
Act
Ethnic media
platforms
English for Speakers
of Other
Languages (ESOL)
programme Centres
Information
Pre vaccination
Vaccination
Post Vacination
• Community/religious
• Some Vaccinators
•
Appropriate culutural
leaders and regional
match the population
and religious
enagagement teams to
•
Appropriate cultural
communiications
Official
support community health
and religious
• Family and community
workers
communiications
leaders support
•
Appropriate cultural and the
• Family and community • Alternative
religious communiications
leaders support
communication formats
• Family and community
•
and channels
leaders support
Alternative access
method
• Safe environment and
• Alternative communication
under •
site set up especially for
formats and channels
Safe environment and
site set up especially
Muslim women
• Safe environment and site
for Muslim women
• Translation service
set up especially for
•
Muslim women
Translation service
• Translation service
Released
21


 Document 3
Document 3
1982
Act
Public Service Workplace
Vaccinations Workshop
Information
Official
the
under
Released

LATEST FACTS AND FIGURES
1982
Act
Information
Official
the
under
Released

WORKPLACES AS VACCINATION SITES
1982
Act
Information
Official
the
under
Released

WIDER ROLL OUT
1982
Act
Information
Official
the
under
Released

TIPS & TRICKS
1982
Act
• Minimum 70 vaccinations per day, ideally 100+
• Site set-up: space for active observation 20 minutes; emergency access
• Challenges of vaccine: super-cold storage, 31 day expiry, 6 hour once drawn up, manage wastage
• Workplace ability to have a ‘reserves list’
Information
• Internal project team, and ‘lead’ for each site
• Privacy
• Key documents: operating guidelines and blueprint
Official
• https://www.health.govt.nz/our-work/diseases-and-conditions/covid-19-novel-coronavirus/covid-19-
the
vaccines/covid-19-vaccine-information-health-professionals#operate
• https://www.health.govt.nz/system/files/documents/pages/workplace-sites-service-design-blueprint-
under
09072021.pdf
Released

WAYS WORKPLACES CAN HELP
1982
Act
Information
Official
the
under
Released

DISCUSSION
1982
Act
Information
Official
the
under
Released



1982
Act
Covid-19 Vaccine and
Information
Immunisation Programme
Official
the
under
Released
 Document 4
Pfizer Covid-19 Vaccination and
Immunisation Programme
Document 4
Pfizer Covid-19 Vaccination and
Immunisation Programme
Workplace model lessons learnt
24 September 2021
1982
Approach
Act
• Due to limited time, resources and the current DELTA lockdown, the planned
evaluation of the Workplace model has been scaled down.
• The purpose of this light evaluation is to identify what has worked well and what has
not, to improve the model prior to a larger group of workplaces coming onboard.
• The insights gathered wil also support future decision making on the size and scope
of the model in relation to wider uptake and CVIP future state.
• The primary areas focused on are;
Information
o Provider capacity
o Pre-engagement
o Invitation strategy
o Booking system and processes
Official
o Reporting
o Equity
o CVIP supporting structures
the
o Considerations for scaling up
• Insights wil be gathered through structured discussions with the Workplace Delivery
team, and workshop with providers and employers.
under
• The Workplace Delivery team wil utilise this document to identify what
issues/recommendations have;
o already been
resolved
o are in the
pipeline
o
need escalating for consideration, or
o are known to
not be feasible.
• This can then be us
Released ed to input into the workplan for improvements.
Page 1 of 8

1982
Provider Capacity
How are providers managing their capacity and reach?
Act
Feedback
Status
Some providers have overcommitted in the EOI process and it is unclear how they wil manage the expectations and scheduling of the
Escalate
workplaces they have committed to.
Having access to NIBs has been critical to demand/capacity planning if using Book My Vaccine. Access needs to be given before
Resolved
workplaces/providers start.
Workplaces prefer to use a walk-in model which may be difficult to capacity plan. Some noted that many staff did not have access to tech to
Escalate
book online and the workplace had to undertake additional tasks to support this i.e. making the bookings for them.
Information
Demand dries up at a point and a decision then needs to be made as to weather it is viable for providers to continue or if staff are then
informed to seek vaccination elsewhere. Some providers are doing half days to accommodate low numbers at the tail end.
There was added work for Mainfreight to create staff contact lists that were then sent to each Branch contact ahead of vaccination events so
they could support staff reminders.
Official
Daily check in’s with providers was critical to track numbers and address capacity issues.
the
Where numbers were low, some workplaces engaged household contacts to fil gaps.
Capacity planning gets impacted when other vaccination sites get located close to the workplace. For example, Auckland Airport Drive-in
was located around the corner from Mainfreight and impacted staff numbers as some chose to head there.
Don’t wait until the end of the day to manage demand issues. One workplace called ‘no-shows’ after 15mins and communicated the
under
importance of not wasting vaccine.
The booking system sent reminders for the first dose but not the second. This led to ‘no shows’ being higher for the second dose. There
was also a problem with duplicate bookings occurring during level 4 lock down. Some workplaces sent mass text reminders to staff to try
and mitigate this as best possible.
Page 2 of 8
Released

1982
Pre-engagement with staff
What activities have been carried out that have led to best possible uptake? How can workplaces support staff to maximise this opportunity?
Act
Feedback
Status
Fisher & Paykel activated engagement with staff a couple of months in advance. As they have an ethnical y diverse workforce, they translated
intel into 5 languages. Info was shared through volunteer staff at vaccination booths set up on campus. There was a direct link between
booths operating and registration numbers.
Mainfreight utilised the Fisher & Paykel material and approach. They had a person on site at each branch. Started with posters. Knowing
your team and knowing how to get them involved is the critical factor, for example knowing when you just need to fil in forms for people to
make the process easy. Did not use incentives as it didn’t fit with their culture. Being able to bring families to also get vaccinated increased
Information
engagement. First big spike occurred when people went back to branch with vaccinated sticker on, prompting others. The DELTA outbreak
drove increased engagement.
Fonterra utilised the Fisher & Paykel and Mainfreight materials and approach and found these really effective. They received advice from
FENZ regarding the value of live virtual Q/A sessions and these worked well. They had medical officers and the global head of Health &
Safety front it which went a long way to make people feel safe. There were lots of individual circumstance questions. Experts are always
critical especially if not an employee or from the programme – more independence the better for webinars. The team curated the questions
Official
as they came through. This approach also helped managed vaccine hesitancy.
Anyone coming into the programme now wil need to consider different engagement tactics as early adopters are likely to already be
Escalate
the
vaccinated. The workplace model may just be targeting those now hard to reach or hesitant. The target audience has now shifted.
It is important for workplaces to have a known position on vaccination to set the tone for their staff.
There are opportunities for workplaces to work collaboratively with other community sites to support staff rather than try and capture the
Escalate
under
minority on-site. Noting that workplaces with shift workers may stil need on-site access as community sites may not be accessible in the
hours they have available. The advantage to hosting on-site is that workplaces can manage shift workers and ensure access to all shifts. If
off-site options were available all hours required, Fisher & Paykel wound not be doing on-site vaccinations in the workplace.
Flexibility was identified as a critical factor to engagement success.
Page 3 of 8
Released

1982
Invitation Strategy
What worked, what were the challenges, how have the roles and responsibilities been applied
Act
Feedback
Status
Workplaces led the invitation approach and activities.
Keep it simple. If people have to do too much work it gets too hard and they opt out.
Some workplaces didn’t invite people to 5 min slots, rather 15 o 30 mins slots to support their planning.
Some workplaces found that emails and texts were not always effective, especially in environments where staff do not have access to
Information
workplace tech or are unable to use tech while working.
Some workplaces delegated invitations to branch contacts in a hub and spoke model. Hub leads had the flexibility to customise to meet
local needs.
Health and Safety staff were utilised in some workplaces to send out all communications regarding the vaccination opportunity.
Official
Fisher & Paykel used their internal call centre that was set up specifically for COVID to contact people who registered interest at their info
booths.
the
When considering pre-engagement and invitation approaches, consider providing to those who want it, convincing those who are hesitant,
and not leaving a large gap between making bookings available as part of the invite and the vaccinations actually starting.
Flexibility is key. Mainfreight found that Friday and Saturdays were most popular so catered for that. The workplace model offers such
flexibility that other delivery models do not. The more flexible the better the outcome.
under
Some workplaces created a deadline as part of the invite approach to encourage early uptake and avoid people leaving it to the last minute.
They used messages like ‘take it or leave it while we have guaranteed supply close to us’, and ‘it’s about keeping everyone around you safe’.
Interest increased when people were given a deadline.
Further clarity and consistency on the consent process including appropriate wording is required.
Escalate
Page 4 of 8
Released

1982
Bookings
What worked, what were the challenges, what would we do differently?
Act
Feedback
Status
Need to either only use Book My Vaccine or internal process, not both. If using Book My Vaccine, workplaces must have access to NIBS.
Resolved
Book My Vaccine is good for individuals but not for groups. People could make last minute changes that impacted demand scheduling.
Escalate
Text reminders were deemed the main/only value of using Book My Vaccine for the Workplace Model. It was noted however, that the text
reminders were inconsistent as some staff did not get them.
The end-to-end admin process is top heavy and could be simplified.
Escalate
Information
Applying the codes is good but the system stil asks for your address to locate the best site which is irrelevant for this model.
Escalate
Mainfreight asked people to indicate how many people were coming together in the car to help understand numbers and balance supply.
Fisher & Paykel – pre-Book My Vaccine, they used an internal booking process and used their internal call centre to contact employees and
Official
give them an appointment. When BMV was utilised, confirmation texts worked well. Overall – having a central group to manage the
interaction and support staff worked well, especially for staff without tech access.
Set up home bubble events over 3 days and used Book My Vaccine for this. Had no ‘no-shows’. Managed walk-ins easily. Eventually
the
moved to a booking from hard copy and consent to make the processes easier.
Mainfreight - Prior to NIBS access – couldn’t see who was actually booked, and people could make changes themselves which caused
chaos. Notifications of bookings were good but people only get it prior to first dose. Worked closely with provider to reconcile booking
schedule daily. When people used Book My Vaccine and out internal process it made it really complicated.
under
Fonterra – Book My Vaccine needs a bulk up-load capability to be useful in the workplace model. Used booking co-ordinators at each hub
(admin).
Page 5 of 8
Released

1982
Reporting
How can we support people, and organisations to have the data they need in a safe way?
Act
Feedback
Status
Access to data in NIBS is critical.
Resolved
If someone from the employer is given concierge access, it would be useful to arrange internal privacy training for them, so their use of
Escalate
identifiable data is appropriate. MoH wil need to provide this if the employer can’t.
Some workplaces had requests from other internal teams wanting access to identifiable data, e.g. HR teams wanting to know who had been
Pipeline
vaccinated so they could reconcile that with leave applications.
Information
Fisher & Paykel captured vaccinations via employees sending in a photo of the vaccination appointment care. But these cards could eb lost
and put through the wash. Need a more efficient way to do this.
Vaccination cards were helpful but have changed and no longer include batch numbers etc.
Official
Equity
Who is being served by this model?
the
Feedback
Status
Serves those who otherwise wouldn’t due to simplified access – already at the workplace site.
• Shift workers and flexible workers are well served by workplace model, also suits those in rural areas
under
• Suits those less tech literate or those who would otherwise not be inclined to
Page 6 of 8
Released

1982
Scaling up considerations
What do we need to stop, start or change to successfully scale up?
Act
Feedback
Status
• By guided by the provider and work closely together
• Flexible and simple
• Know your team and what works for your people
• Lockdown and delta – people have been scared about members going to work but by including family/whānau it increased
confidence/ feelings of safety in going to work.
Other
Information
What have been the intended and unintended consequences if any?
Feedback
Status
• Lot of gratitude from workers, particularly ahead of age bands
• Peer pressure has been mostly positive for breaking down barriers
Official
• People who weren’t for it, made calls to book anyway
• Workplaces thinking about mandatory vaccination in future
the
• Time off for AEFI doesn’t impact leave
• Anti-vaxxers are in the minority, most convos happened at branch level.
Operating Guidelines are stil DHB centric and causing confusion
Escalate
CVIP needs established systems and processes for stakehol
under der management for Occupational Health and Central Government.
Escalate
Consider an Advisor from the Occupational Health sector to join the team and support sector relationships.
Escalate
Page 7 of 8
Released

1982
Supporting Structures
What has worked well, what are the challenges? From the Workplace Delivery Team’s perspective
Act
Feedback
Status
The shared support service approach of other teams is not assisting in solving the problem due to other priorities, capacity, and capability.
The Workplace model is unique in that MoH is directly commissioning national providers to complement the DHBs, and it requires a 3-way
relationship between commissioning agent, provider and employer. This means a specific small team is established to manage the
national delivery within CVIP. No other service model requires this approach. The implications of this being the only model to have this
approach, is that the structure of key enabling teams, systems and processes are primarily designed to serve DHB delivery. Accessing
key teams to support the end-to-end implementation process has been difficult due to competing priorities and unknown capacity of other
teams to support critical process like provider onboarding.
Information
The Workplace delivery team has by default become the ‘shop front’ for providers and employers without the resource required to do that
Escalate
as best as possible, impacting the focus they need to have on running the processes well. If this cannot be addressed the RAM resource
requirement wil need to increase.
There is no dedicated budget to support the team. Steering Group requests are being made to seek agreement on spend. This is not time
Escalate
efficient and a new approach with the Programme Office should be sought.
Official
The tool built to identify bottle necks in the end-to-end process has been invaluable.
the
under
Page 8 of 8
Released
Document Outline
- HNZ00049846 annex one.pdf































