

5 August 2024
Catherine Jamieson
[FYI request #27405 email]
Tēnā koe Catherine,
Your request for official information, reference: HNZ00054972
Thank you for your email on 9 July 2024, asking Health New Zealand | Te Whatu Ora about early
Covid vaccine distribution systems and data under the Official Information Act 1982 (the Act):
1. the name of the system(s) that held vaccine distribution data prior to the commissioning/full
commissioning of the Covid Immunisation Register;
2. what part of what agency was responsible for that system or systems; and
3. information to demonstrate how the functions of the Inventory Portal that sits within the
Covid Immunisation Register were carried out before the Inventory Portal and CIR were (fully)
commissioned.
Is this information subject to the Public Records Act?.
Response
For the sake of clarity, I will answer each question in turn.
1. the name of the system(s) that held vaccine distribution data prior to the commissioning/full
commissioning of the Covid Immunisation Register;
The
National Final database held vaccine distribution data before implementation of the COVID-19
Immunisation Register (CIR). Since December 2023, the Aotearoa immunisation register (AIR) has
replaced CIR and National immunisation register (NIR).
2. what part of what agency was responsible for that system or systems; and
The Ministry of Health’s COVID-19 Vaccine Immunisation Programme was initially responsible for
this database. Following establishment of Health NZ, the responsibility transitioned to the National
Immunisation Programme, and then to Prevention (National Public Health Service).
3. information to demonstrate how the functions of the Inventory Portal that sits within the
Covid Immunisation Register were carried out before the Inventory Portal and CIR were (fully)
commissioned.
Please see attached
Appendix 1 Customer Service – How to place an order using the Planning Tool and
Appendix 2 8a. Customer Service – Order Processing – Vaccines.
Please note that personal details (mobile numbers) of some individuals have been withheld under
section 9(2)(a) of the Act to protect privacy. In making this decision, we have considered the
countervailing public interest in release and determined that it does not outweigh the need to withhold
on this occasion.


 Is this information subject to the Public Records Act?
Is this information subject to the Public Records Act?
Yes, this information is covered by the Public Records Act 2005 (PRA). Both the Ministry of Health
and Health NZ (which includes former entities known as District Health Boards) are considered public
offices under the PRA.
How to get in touch
If you have any questions, you can contact us at
[email address].
If you are not happy with this response, you have the right to make a complaint to the Ombudsman.
Information about how to do this is available at
www.ombudsman.parliament.nz or by phoning
0800 802 602.
As this information may be of interest to other members of the public, Health NZ may proactively
release a copy of this response on our website. All requester data, including your name and contact
details, will be removed prior to release.
Nāku iti noa, nā
Sara Freitag
Manager, Machinery of Government Support
National Public Health Service
TeWhatuOra.govt.nz
Health NZ, PO Box 793,
Wellington 6140, New Zealand


Example – Material created for training purposes
APPENDIX 1
Order Processing – Vaccines
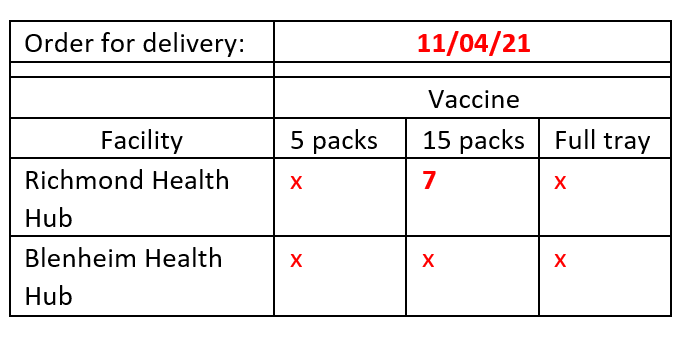
1. Orders are received via the COVID-19 Logistics inbox in the following format. These must be
received by 10am the day prior to delivery. The email will have a subject line of VACCINE
ORDER – XXX.
1982
2. If vaccine orders are received after 10am, the SRO at the relevant DHB must approve this
before the order can be processed.
the Act
3. As orders are received, an order form should be created in Teams for the required delivery
under
date (copy a prior order form and clear out irrelevant information). Verification of emails
should be copied into the order form into a new tab named with the relevant DHB.
Complete this process for each individual order.
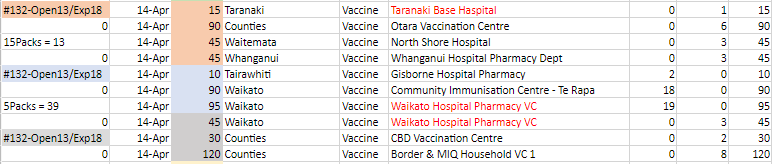
4. Once all orders are received and confirmed (10am the day prior to delivery), the transport
Information
plan should be drafted with all current orders that have been recorded in the draft order
form. These orders are then colour categorised based on what ULT tray they are being
fulfilled from (example below). Leftover stock from trays already opened should be allocated
first, then additional tray repacks required should be planned out in the transport plan.
Released
Official


Example – Material created for training purposes
5.
10:30 meeting – vaccine and consumables sign off. In this meeting, the transport plan is
reviewed by the wider team and the number of tray repacks to be sent to HCL is confirmed
through discussions.
6.
10:45 - tray repack request for the day is drafted and sent to HCL. (Currently 2 trays are
already being broken down each day by HCL, so the repack request is for any trays required
on top of this).
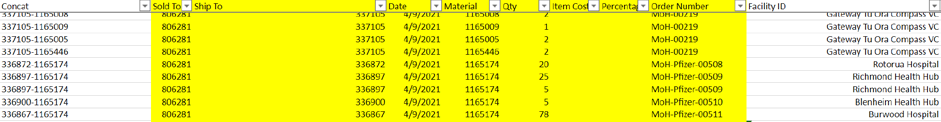
7. Once the repack request is sent, the order form to be sent to HCL is completed (example
below).
s9(2)(a)
8.
11:15 – meeting with NZPost regarding next day deliveries.
9.
12:30 – Full vaccine order form due to be sent to HCL and NZPost.
1982
10.
13:00 – 1pm confirmation of confirmed vaccine orders for the following day is to be sent to
DHB Logistics Leads and RAM’s.
the Act
under s9(2)(a)
Information
Released
11. Order register in National Final to be updated with vaccine orders.
Official
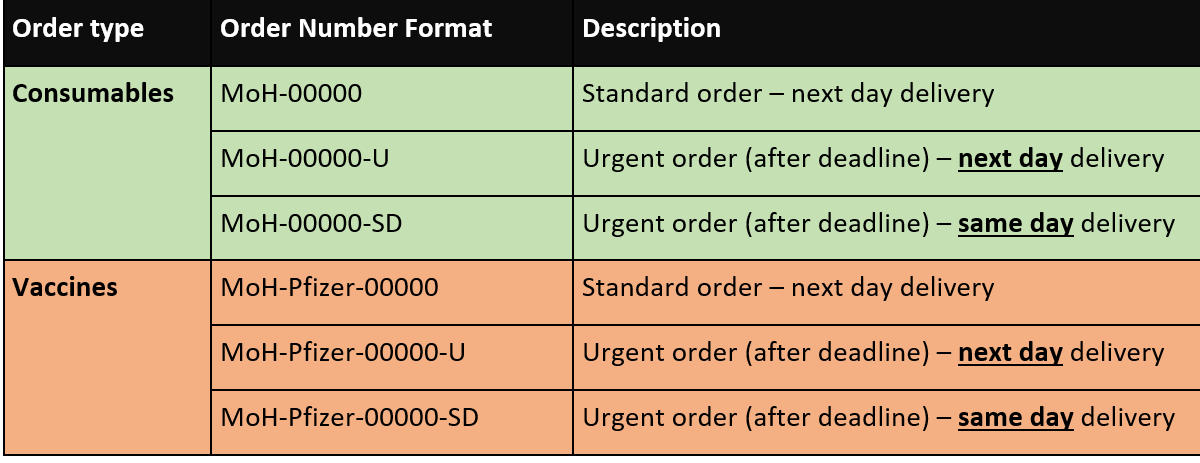
Order numbers are to be formulated using the below:
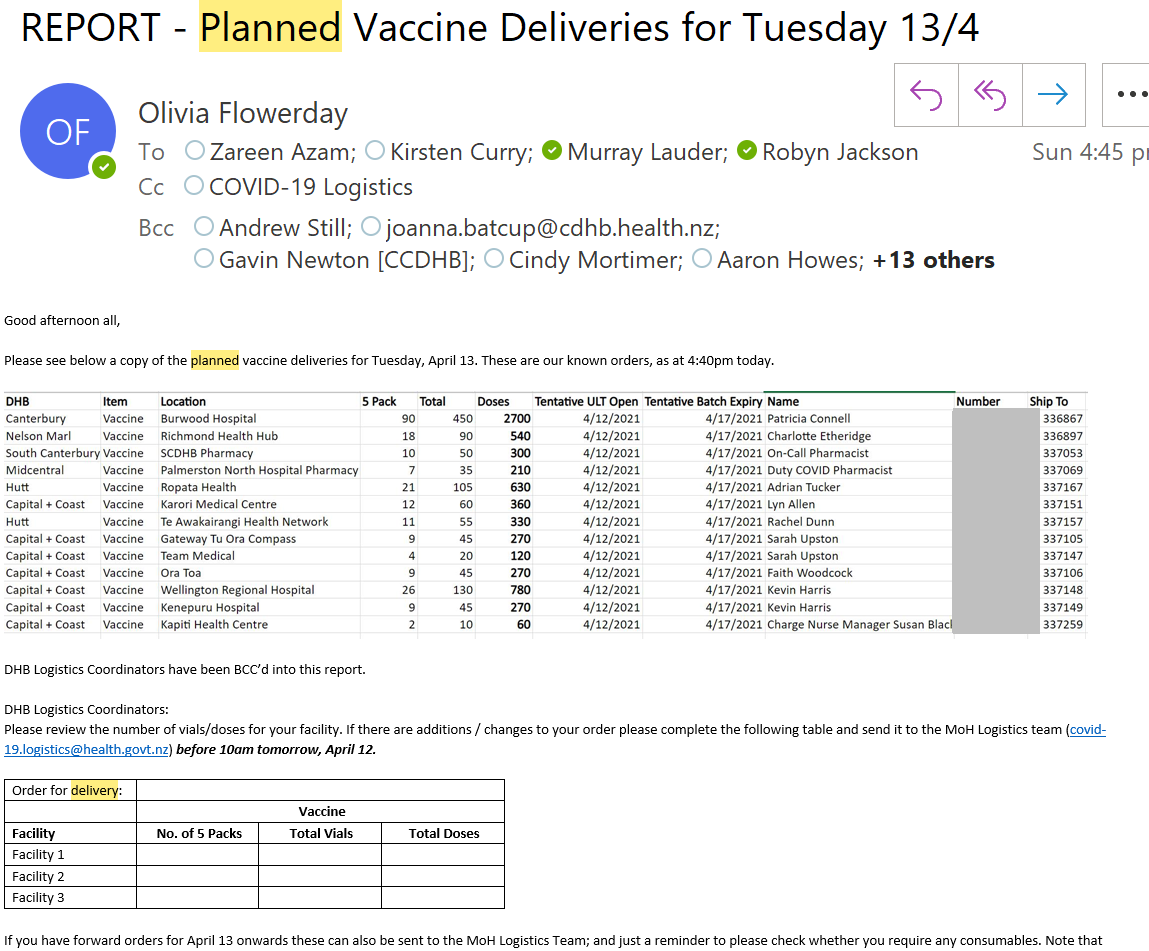
Example – Material created for training purposes
12. Orders 48 hours out are then entered into transport plan using what verifications have
already been placed in the order form for that specific day.
1982
13.
17:00 – 5pm confirmation of orders for 48 hours out is sent to RAM’s and DHB Logistics
Leads. This is also sent to NZPost as a ‘Tentative Transport Plan’.
the Act
under
s9(2)(a)
Information
Released
Official

 APPENDIX 2
How to place an order using the Planning Tool
1. Record the vaccine orders that have been requested.
APPENDIX 2
How to place an order using the Planning Tool
1. Record the vaccine orders that have been requested.
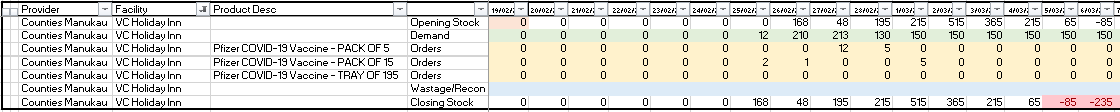
a. The yellow rows contain all placed orders. To plan an order, overtype the formula
with required orders. In the below example 5 packs of 15 have been planned for 1st
March delivery. This pushes out the next required order to the 4th March.
2. Create an order.
a. In the Order Form – Vacc tab, select the date and use the filter to select the facility.
b. This wil auto populate the information required. This can then be sent to HCL for
processing.
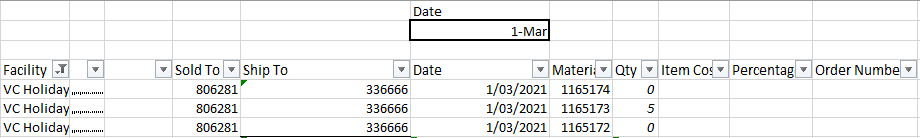
3. Capture the order.
a. Once the order is sent to HCL it needs to be added to the master list of orders. In the
OR tab, paste in the HCL order info into the yellow cells.
under the
b. In the OP tab, right click and refresh the pivot table.
c. Copy paste the whole table into the tab OF.
4. Pull through placed orders.
a. Back in the Proj – Vaccine tab, drag across the formula in the orders rows so that the
manual text you planned earlier is replaced by the formula. If the order has been
loaded correctly, the formula should bring through the order volume.
Released
Official Information Act 1982
















