 Priority
Priority – Low
Security Level – In Confidence
To: Hon Todd McClay, Minister of Agriculture
From: Sothea Tep, Manager Horticulture Sector Policy
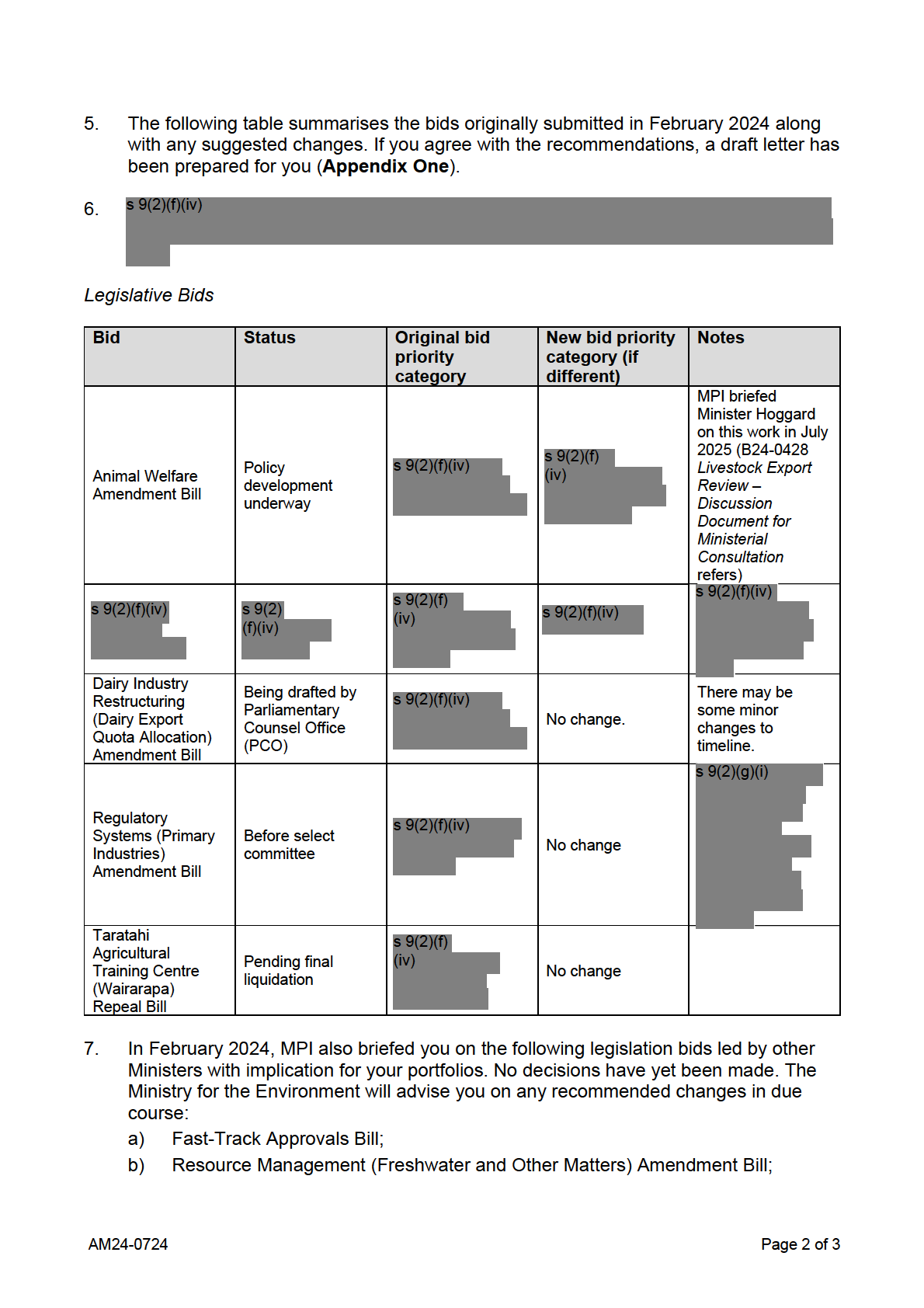
Impact on the kiwifruit industry from the Environmental
Protection Authority’s new controls for hydrogen cyanamide
Date 30 May 2024
Reference
AM24-0522
Purpose
•
This aide-memoire provides you with information on the impacts to the kiwifruit
industry from the Environmental Protection Authority’s (EPA) decision to retain the
approval for hydrogen cyanamide1 with new controls.
•
You may wish to forward this paper onto the Associate Ministers of Agriculture.
New controls for hydrogen cyanamide are likely to have a low impact on kiwifruit
growers in the long term, but there may be near term challenges
1.
Overal , the EPA’s decision to retain the approval for hydrogen cyanamide is positively
received by the kiwifruit industry as they will continue to have access to this important
agrichemical to promote budbreak (mimicking the effect of winter chil ing) for kiwifruit
to develop properly, uniform production and efficient harvesting.
2.
Having continued availability of hydrogen cyanamide also has flow on benefits as it
provides more certainty for growers and potential investors to invest in the kiwifruit
industry. The kiwifruit industry body New Zealand Kiwifruit Growers Incorporated (NZKGI)
has suggested the uncertainty of hydrogen cyanamide’s future during the EPA’s
reassessment muted investment into the industry.
3.
The new controls on hydrogen cyanamide are likely to have low impacts for the
kiwifruit industry overall. Impacts will vary between individual growers (due to their
orchard setup) and the region they are based in (due to variability in winter chil ing).
Growers in regions with warmer winters or have orchards near waterbodies or non-
target threatened plant species are likely to be more impacted.
Appendix One details
the likely effects of the different controls for hydrogen cyanamide on growers.
Official Information Act 1982
1 A commonly used brand of hydrogen cyanamide is ‘Hi-Cane’.
AM24-0522
Page 1 of 3
4. The new controls are not a surprise for the industry as they were also consulted,
addressed, and amended through the EPA’s reassessment and hearing process.
The new controls are considered by industry to be less concerning and more workable
than the prospect of a potential phase out and ban of hydrogen cyanamide.
5. NZKGI has highlighted a challenge for the industry wil be educating and giving
assurances to the community on hydrogen cyanamide use. The EPA’s reassessment
of hydrogen cyanamide brought attention to the risks and impacts of its use. The
industry wil be looking to assure their communities that they use hydrogen cyanamide
safely with best practices.
6. Hydrogen cyanamide is a minor agrichemical used on other crops including apple,
1982
cherry, apricot, and kiwiberry. Growers in other industries may find it difficult to comply
with the new controls, but they are less reliant than the kiwifruit industry on using
hydrogen cyanamide as alternatives are available2.
EPA’s new controls on hydrogen cyanamide take immediate effect
Act
7. On 23 May 2024, the EPA’s Decision-Making Committee decided to retain the
approval for hydrogen cyanamide, but with new controls that take effect immediately
(except controls for labelling and packaging in which are in effect from 1 July 2025).
8. New controls were required due to the need to mitigate risks to operators, bystanders,
the aquatic environment, non-target plants, soil organisms, birds, pollinators, and non-
target arthropods.
9. New controls for hydrogen cyanamide and their effects on growers are detailed in
Appendix One, including:
a) application can only be made once per year between 1 July and 10 September;
b) maximum application rates (with different rates for kiwifruit versus other fruit);
c) buffer zones to protect bystanders, the aquatic environment, and non-target
plants downwind of the target plants;
d) application limited to ground-based methods, with nozzles that produce coarse
or larger droplets being permitted for use;
Information
e) a maximum wind speed restriction;
f)
changes to labelling and packaging requirements (takes effect 1 July 2025); and
g) qualification requirements for professional users.
10. The EPA did not consider setting requirements for personal protective equipment (PPE)
as that is the responsibility of WorkSafe.
Official
2 For example, Hi-Cane alternatives available to apple growers include Erger® and Waiken™.
AM24-0522
Page 2 of 3
11. There are likely to be consequential changes to hydrogen cyanamide based products
registered under the Agricultural Compounds and Veterinary Medicines Act 1997.
New Zealand Food Safety is reviewing the possible changes needed and wil contact
registrants on this matter.
Minister / Minister’s Office
Seen / Referred
/
/ 2024
Official Information Act 1982
AM24-0522
Page 3 of 3

1982
Act
Information
Official

1982
Act
Information
Official
13. This is unlikely to have a significant impact on growers, as it is a mat er of having
appropriate application equipment. Some growers may need to upgrade their
equipment. Many growers contract third parties to apply hydrogen cyanamide; they
may also need to upgrade their equipment.
Maximum wind speed restriction of 20 kilometres per hour
14. This is not likely to impact growers, as they already do not apply hydrogen cyanamide
in windy conditions with 20 kilometres per hour around the top end of industry
recommendations.
Label ing and packaging requirements
1982
15. New labelling and packaging requirements mainly consist of updating the hazardous
substance labelling (for example, hydrogen cyanamide now being classified as
corrosive rather than just an irritant), safety instructions and application instructions
(for example, users should be aware of any wetlands, indigenous vegetation or
Act
reserves that may contain threatened plants adjacent to the application area).
16. These changes are not likely to impact growers’ ability to grow kiwifruit, however, may
have some impact in terms of health and safety in using hydrogen cyanamide and
compliance with the new controls as this requirement only comes into effect by 1 July
2025.
Qualification requirements for users
17. The qualification requirements require users to be qualified to handle substances
classified as being hazardous to the aquatic environment, with requirements outlined
under the Hazardous Substances (Hazardous Property Controls) Notice 20174.
18. This may have some impacts on growers if they are not already qualified to handle
hazardous substances. Other agrichemicals often require qualifications to handle, so
many growers may already be qualified or wil be contracting qualified third parties.
Information
Official
4 https://www.epa.govt.nz/assets/Uploads/Documents/Hazardous-
Substances/GHS2/Consolidated_Hazardous_Substances_Hazardous_Property_Controls_Notice_2017.pdf
AM24-0522
Page 3 of 3
Appendix One
 Priority
Priority – High
Security Level – In Confidence
To: Hon Todd McClay, Minister of Agriculture
Hon Andrew Hoggard, Minister for Biosecurity, Minister for Food Safety
Hon Mark Patterson, Minister for Rural Communities, Associate Minister of
Agriculture
From: Fiona Duncan, Director Regulatory Systems Policy
1982
Gene Technology Regulation Reform: Legislative Purpose and
the Regulator
Act
Date 7 June 2024
Reference
AM24-0475
Purpose
•
This aide-memoire provides information on key policy decisions that wil be taken at
the Ministerial Group meeting on 11 June 2024. The Ministerial Group is being used to
finalise policy decisions before Hon Judith Collins KC, Minister of Science, Innovation
and Technology, takes a paper to Cabinet on 25 July 2024.
Background and Context
1. The Government has committed to the reform of New Zealand’s gene technology
regulations and legislation (the reform) and this process is now underway. As a
member of the Ministerial Group on gene technology reform, you have been invited to
a meeting on 11 June 2024.
2. The Ministerial Group has met twice to date to discuss aspects of the reform process
and has agreed on the timeline, approach, scope, objectives1, risk tiering framework,
Information
exemptions and definitions for the reform.
3. A list of aides-memoire you have previously received to support Ministerial Group
Discussion is supplied in
Appendix One.
4. At this third meeting, we expect delegations, the legislative purpose, the form and
location of the new regulator, and compliance monitoring and enforcement to be
discussed.
Official
1 At the most recent meeting on 8 May 2024, Ministers agreed to the development of an additional objective
on international alignment.
AM24-0475
Page 1 of 5
Policy decisions to be made
Legislative purpose
5. The purpose of the Hazardous Substances and New Organisms Act 1996 (HSNO Act)
is to protect the environment and the health and safety of people, by preventing or
managing adverse effects. It also requires specific principles and matters to be
provided for, such as safeguarding the life-supporting capacity of ecosystems, Māori
cultural values and economic benefits and costs.
6. The new gene technology regulation is being modelled on the Australian regulatory
system, which is based on their Gene Technology Act 2000. This Act has a similar,
1982
but more targeted purpose and basis for decision-making, compared to the HSNO Act.
Specifically, its purpose is to
“protect the health and safety of people, and to protect
the environment, by identifying risks posed by or as a result of gene technology, and
by managing those risks through regulating certain dealings with GMOs.”
Act
7. The Ministry of Business, Innovation and Employment (MBIE) is recommending the
new legislation’s purpose be based on the Australian system and focus on risk
management rather than prevention, and only in the key areas of human and
environmental health and safety.
8. There are arguments on both sides for the inclusion or exclusion of additional criteria
for consideration by the new regulator.
a)
Inclusion can provide mechanisms to weigh up potential benefits against
potential costs to specific sectors, including primary producers and protect
organic, halal and other gene technology-free food production systems.
However, it can also lead to increased approval times and costs for applicants
and the new regulator; and
b)
Exclusion could reduce costs associated with decision making for both
applicants and regulators, but may have unintended consequences for others
such as New Zealand’s primary sector – for instance, where significant benefits
of an application to the sector are unable to be considered by the new regulator.
Information
9. The Ministry for Primary Industries (MPI) is supportive of the legislative purpose
focussing on risk identification and proportionate management, to achieve the agreed
reform objectives.
Delegations
10. The new gene technology legislation wil introduce new requirements, risk assessment
and decision processes, and approvals.
11. MBIE recommends that the new legislation enable the regulator to delegate some of
its powers to other regulatory agencies, when it is more appropriate for a single
regulator to make that decision. This could include preparing a risk assessment or
Official
management plan, making a decision and set ing conditions for activities involving
genetically modified organisms (GMOs).
AM24-0475
Page 2 of 5
12. The reform also proposes that the new regulator be given the ability to undertake joint
assessments of applications with other overseas regulators, while stil retaining the
ability to make independent decisions based on the joint review. The ability to make
independent decisions is essential to account for New Zealand’s unique context and
environment. This includes the ability to accept an international risk assessment, and
adapt it for New Zealand’s context instead of conducting a full-scale assessment.
13. The regulator would be given the ability to assess certain applications through an
expedited pathway where an application has previously been assessed by a
‘recognised’ regulator.
14. MPI supports the proposal for the new regulator to have power to delegate. Similar
provisions are already in operation - for example, currently all foods produced using
gene technology are assessed by Food Safety Australia New Zealand (FSANZ) before
they can be sold in either country.
Form and location of the new regulator
15. A new gene tech regulator is proposed as part of the gene technologies reform
process. There are different options for the form the regulator could take, with different
levels of independence. You have the opportunity to consider which one would
provide the most efficient administration of new gene technology regulation.
16. The regulator was originally
envisaged in the form of a departmental agency with
MBIE as the host agency. However, the Public Service Commission has indicated it
will not support the establishment of a new entity (departmental agency or Crown
entity) for this role.
s 9(2)(g)(i)
18. Different organisational structures, including degree of ministerial oversight, are
outlined in
Appendix Two.
s 9(2)(g)(i)
Official Information Act 1982
AM24-0475
Page 3 of 5
s 9(2)(g)(i)
1982
Act
Information
Compliance monitoring and enforcement
25. The establishment of a new regulator provides the opportunity for you to discuss how
compliance, monitoring and enforcement (CME) should be carried out in the future.
This includes whether to continue having separate organisations in charge of
administration and compliance or to consolidate these functions. It is important to
consider the appropriate resourcing and allocation of duties that would be required for
implementation of this decision.
Official
AM24-0475
Page 4 of 5
26. In the Australian system, the Office of the Gene Technology Regulator (OGTR), is a
‘one stop shop’, including operating its own CME team. The responsibilities of the
team include approving places to conduct GMO activities, compliance monitoring
those places, inspections and monitoring compliance with GMO licence conditions and
undertaking investigations through to prosecution.
27. In New Zealand MPI is the statutory enforcement agency for genetically modified
organisms (as new organisms) under the HSNO Act in respect to new organisms,
which includes GMOs by definition. MPI undertakes CME activities pertaining to gene
technologies, including those the OGTR is responsible for in the Australian system,
using provisions under both the HSNO Act and other Acts (such as the Biosecurity Act
1993).
s 9(2)(g)(i)
Next Steps
31. The fourth Ministerial Group meeting is expected to be held during the week of 24
June 2024. We expect this meeting to cover the topics of Māori rights and interests,
interactions with other agencies and statutory bodies, national consistency, CME and
penalties. We wil provide you an aide memoire ahead of the next Ministerial Group
meeting.
32. An aide memoire is being developed on consumer preferences and the market risks of
reforming New Zealand’s gene technology legislation and regulation. This wil include
insights and implications for the primary sectors.
Official Information Act 1982
Minister / Minister’s Office
Seen / Referred
/
/ 2024
AM24-0475
Page 5 of 5
Appendix One: Aides-memoire you have previously received
Title
Reference
Received by
An Overview of Genetically Modified
AM24-0019
Minister for Food Safety
Foods
Gene technology Ministerial Group
AM24-0265
Minister of Agriculture
Meeting
Minister for Biosecurity
Minister for Food Safety
Interfaces of the Hazardous
AM24-0409
Minister of Agriculture
Substances and New Organisms Act
Minister for Biosecurity
1996, Biosecurity, Agriculture, and
Minister for Food Safety
1982
Food portfolios
Associate Minister of Agriculture
(Animal Welfare, Skills)
Genetic Technology Regulation
AM24-0410
Minister of Agriculture
Reform: Background & Scope
Minister for Biosecurity
Minister for Food Safety
Minister for Rural Communities
Act
Associate Minister of Agriculture
Gene Technology Ministerial Group
AM24-0449
Minister of Agriculture
Meeting – 8:00pm 8 May 2024
Minister for Biosecurity
Minister for Food Safety
Minister for Rural Communities
Associate Minister of Agriculture
Information
Official
AM24-0475
Page 1 of 1
Appendix One

 Appendix Two: Government agency structure diagrams
Appendix Two: Government agency structure diagrams
Al diagrams are from the Public Service Commission, which can be found on the website
here: https://www.publicservice.govt.nz/
Figure 1: Types of Government organisation and degree of Ministerial influence
Official Information Act 1982
Table 1: Type of Government organisation and decision making powers
AM24-0475
Page 1 of 1
Appendix Two
 Priority
Priority – Medium
Security Level – In Confidence
To:
Hon Todd McClay, Minister of Agriculture
From: Jenny Cameron, Chief Transformation Officer
Update on Double Export Value Eight-Point Plan
Date 7 June 2024
Reference
AM24-0569
Purpose
•
This aide-memoire provides you with an update on the Ministry for Primary Industries’
(MPI’s) operational planning to enable the food and fibre sector to Double Export
Value over the next ten years.
MPI’s eight-point plan to Double Export Value focuses on key settings to unlock growth
potential for the food and fibre sector.
1.
MPI’s operational planning provides a focus on delivering the Government’s goal of
doubling New Zealand’s export value in ten years. An eight-point plan was developed
after a strategic planning process (attached at
Appendix One).
2.
In order to understand what would unlock more value from exports and increase
export intensity, we listened to sector and business stakeholders, analysed thinktank
perspectives, and undertook our own market and economic analysis.
3.
We focused on eight target areas to enable the settings to unlock growth across the
food and fibre sectors:
a)
maximising trade opportunities;
b)
investment for growth;
c)
enabling regulatory settings;
d)
smarter ways of working;
e)
infrastructure for growth;
f)
championing our attributes;
g)
innovation to create value; and
h)
Māori food fibre value growth.
Official Information Act 1982
AM24-0569
Page 1 of 2
Doubling export value can only be achieved with extra strategic effort to bump forecasts
beyond its current growth trajectory.
4.
Situation and Outlook for the Primary Industries (SOPI) analysis shows export value
growth for New Zealand’s primary industries is forecast to steadily increase, with
continued international demand for New Zealand’s primary products over the decade.
5.
Additional effort is needed to realise the national ambition to double export value over
a decade. This requires a rise from an average 4.7 percent compound annual growth
rate (CAGR) which has been achieved over the last decade to 7.2 percent CAGR over
the next decade.
The eight-point plan is deliberately focused on the areas within the Ministry for Primary
Industries’ influence, expertise, and elements that are most critical for the food and fibre
sector.
6.
The plan focuses on MPI’s area of influence and expertise to reduce costs for
business, remove friction in the system, assist more connection and information to
enable exporters, and to unlock growth potential. The intention is to enable the
settings to allow exporters to realise more opportunities and deliver growth through
business.
7.
The eight-point plan will form part of MPI’s operational plan and strategic priorities,
which will be developed into a work programme over the next two months. The Double
Export Value growth plan is a significant rallying call for MPI, which is already
harnessing the energy, initiatives, and prioritisation of resources to support primary
sector businesses towards the goal.
8.
The work will be overseen by the Director-General, who will act as the Governance
chair, along with his Senior Leadership Team. The Director-General will update you on
the progress of the plan throughout the year.
9.
As the Government’s own economic growth plans develop, we will flex this
Operational Plan to maintain alignment of effort.
Minister / Minister’s Office
Seen / Referred
/
/ 2024
Official Information Act 1982
AM24-0569
Page 2 of 2
Appendix One: Double Export Value Eight-Point Plan
This document is withheld in full pursuant to section 9(2)(g)(i) of
the Act - to maintain the effective conduct of public affairs
through the free and frank expression of opinions by or between
or to Ministers of the Crown or members of an organisation or
officers and employees of any public service agency or
organisation in the course of their duty.
1982
Act
Information
Official
AM24-0569
Page 1 of 1
Appendix One

1982
Act
Information
Official
1982
Act
Information
Official

1982
Act
Information
Official
Appendix One: Draft letter to the Legislation Coordinator, Cabinet Office
Official Information Act 1982
AM24-0724
Page 1 of 1
Appendix One

1982
Act
Information
Official

1982
Act
Information
Official

1982
Act
Information
Official

1982
Act
Information
Official













