1982
Act
Information
Official
the
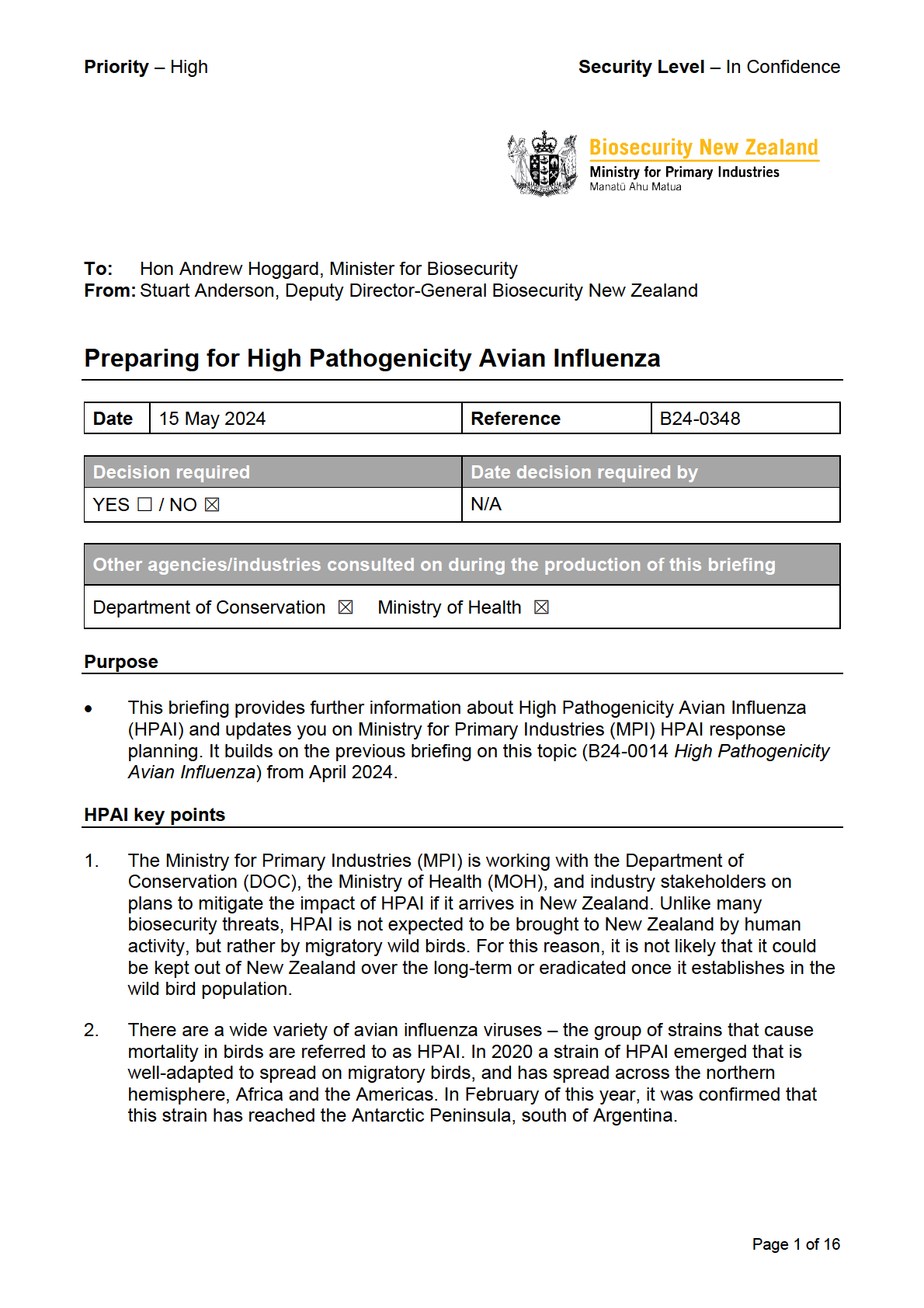
under
Released
B24-0348
3.
HPAI can impact conservation species, waterfowl, small-scale poultry flocks and
commercial poultry production. HPAI can also spill over from birds to mammals, with a
small number of human cases recorded. Multiple other mammal species have been
infected, with cattle in the United States (US) being the most recent finding (Paragraph
39 refers).
4.
HPAI is not present in New Zealand, Australia, or the South Pacific. It has recently
reached the Antarctic peninsula and is expected to spread across the Antarctic land
mass and reach the Ross Sea Region south of New Zealand. The timing of this
1982
progression is unclear, but estimates suggest that this could occur during the next 12
months. As the disease spreads, the risk of it entering New Zealand increases.
Act
5.
Impacts on the poultry sector, especially on free-range producers with limited poultry
housing, are expected to be severe. Rigorous on-farm biosecurity is successfully used
overseas to protect commercial poultry from infection, but it is not possible to fully
protect free-range poultry from infection by wild birds. In the United Kingdom (UK), the
government put in place a mandatory order requiring all poultry to be housed indoors
from November 2022 – this was progressively lifted on a regional basis through the
first half of 2023.
6.
The implementation of rigorous on-farm biosecurity in New Zealand now, before an
incursion, has the potential to protect individual farms from infection and to limit
Information
impacts on the domestic supply of poultry meat and eggs. MPI’s response will support
the sector in adoption of safe practices to meet the response objectives, safeguard
human and animal welfare, and manage Crown liability under the Biosecurity Act
1993.
7.
The impacts on wild birds and marine mammals may be significant but cannot be
quantified in a New Zealand context as highly variable impacts have been observed in
Official
other parts of the world.
8.
The World Health Organization currently assesses the ‘overall public health risk posed
the
by HPAI to be low, and for those with exposure to infected birds or animals or
contaminated environments, the risk of infection is considered low to moderate.
9.
Clear and aligned messaging from Ministers and relevant agencies will be required in
the event of an incursion. Impacts on biodiversity, agriculture, and trade are expected
under
to arise. There is also the possibility of human health impacts, but these have not
been a major issue at the population level with this strain to date.
HPAI response strategy
10. As outlined in briefing B24-0014 (
High Pathogenicity Avian Influenza), it is unlikely that
national eradication will be feasible due to both the likelihood of re-introduction by
migratory birds and the impossibility of eradicating the virus in wild bird populations.
Released
11. Impacts on the poultry industry are best managed by the poultry industry implementing
farm-specific biosecurity measures, supported by MPI.
Page 2 of 16
B24-0348
12. The objectives for a HPAI response are to:
a)
reduce the impact on native species and biodiversity;
b)
reduce the impact on the poultry sector;
c)
maintain supply of poultry meat and eggs to the domestic market and maintain
access to overseas markets where possible; and
d)
protect human health.
13. A One Health approach is being adopted as this strain of HPAI has potentially
significant impacts on the poultry sector, wildlife and biodiversity, and public health. 1982
One Health is “a collaborative, multisectoral, and transdisciplinary approach … to
tackle complex health challenges at the human-animal-environmental interface”. MPI,
DOC and MOH are working together to develop response options that represent the
Act
best approach for New Zealand to protect our unique native species, mitigate the
impact on the poultry sector and take preventative measures to protect human health.
14. MPI is drafting a Cabinet paper for your consideration, setting out some decisions that
could be taken in advance of an incursion. This would clarify the expectations of
different parties around the nature and scope of Crown involvement and the
responsibilities of the relevant agencies and industry. Following receipt of this briefing,
we will discuss these options and the framing of the Cabinet paper with you.
New Zealand context
Information
15. To ensure the continuation of their international exports of poultry commodities,
Canada, the US and the UK currently use legal powers to limit spread of disease. This
ensures they can return to freedom in poultry according to the World Organisation of
Animal Health (WOAH) code chapter on HPAI. The use of legal powers has come at a
substantial compensation cost for these countries, with compensation in the US
Official
reaching USD $500 million in 2023 alone.
16. UK officials have shared their observation that as endemic stability is reached in wild
the
birds, fewer spillover events are occurring into domestic poultry. Despite the
decreased infection pressure, continued biosecurity measures to limit contact between
commercial poultry and wild birds are required. This decrease in spill-over events from
wildlife to poultry is only occurring now in the 4th year of their response to this strain.
17. With a small domestic-focused poultry sector and limited exports of poultry genetics
under
and commodities, the options for how New Zealand best responds to an incursion of
HPAI into poultry differ from those of the countries mentioned above.
18. Figures from 2022 show that New Zealand produced over 119 million chickens from
151 farms, largely for the domestic market. The value of the chicken meat industry to
New Zealand in 2022 was roughly $1.8 billion, with international trade accounting for
only a small percentage of this figure.
Released
Page 3 of 16

1982
Act
Information
Official
the
under
Released
B24-0348
9(2)(g)(i)
1982
Act
Building Poultry Industry Resilience to an HPAI Incursion
26. An adjustment to the New Zealand way of poultry farming to protect domestic supply
of commodities is a sustainable way to prepare for an incursion of HPAI into New
Zealand with wild birds over the long term. This will in turn enhance New Zealand’s
domestic poultry food security and resilience to any future biosecurity threats.
Information
27. Significant biosecurity measures have been identified as effective at preventing
infection of poultry by wild birds in affected countries. These include requiring use of
footbaths and clothing changes when staff move between production houses, regular
disinfection of equipment and hard surfaces, and preventing wild bird access to
supplies of litter and feed. In late 2022 the UK also imposed a compulsory housing
order requiring all poultry to be kept indoors – this remained in place for approximately
six months in most regions until infection pressure decreased. The implementation of
Official
increased biosecurity measures in New Zealand before an incursion has the potential
to protect individual farms from infection, and to limit impacts on the domestic supply
of poultry and eggs.
the
28. MPI is working with the poultry industry to enhance their resilience in the face of a
prolonged HPAI incursion. This will be the primary focus for readiness activities
relating to the poultry industry over the coming months. The initial focus is to work with
industry to understand the current state of biosecurity preparedness, industry
under
experience overseas, and provide advice and guidance to minimise on-farm risk.
29. Initial indications suggest that larger commercial enterprises will be well-equipped to
limit biosecurity risk of HPAI. The smaller enterprises, especially free-range farms with
limited housing, may struggle to limit their exposure risk.
30. An opportunity to support the sector in adoption of safe practices will help protect the
industry, safeguard human and animal welfare, and manage future liability under the
Biosecurity Act.
Released
Page 5 of 16

1982
Act
Information
Official
the
under
Released
B24-0348
Impacts on Mammals
38. The current strain of HPAI has been reported in a range of other mammals, including
goats, dogs, cats, pigs, bears, skunks, racoons and mink, plus marine mammals
including dolphins, seals and sealions.
39. Infected mammals generally live in close contact with dense bird populations and/or
consume infected birds.
1982
40. A multi-state outbreak (9 states, 42 infected herds) of HPAI in dairy cows was first
reported on 25 March 2024 in the US, originating in Texas. This is the first time HPAI
has been found in cattle. While still related to the globally dominant circulating strain of
Act
HPAI, this genotype has only been found in the US, and to date only in lactating dairy
cattle. No evidence of any HPAI infection in cattle has been observed anywhere
outside of the US, despite HPAI being well established in European countries with
large dairy industries.
41. US federal officials have instituted a testing programme for dairy cattle moving
between states to limit further spread of the event. The spillover event from wild birds
to cattle in the US is highly likely to be the result of particular environmental and
production circumstances.
Information
42. Should such an event occur in New Zealand, our legal framework and existing work to
ensure the continuity of processing of milk in the event of an Foot and Mouth Disease
outbreak puts us in a strong position to institute targeted measures at individual farm
level to stop product from affected animals entering the supply chain. Regular
discussions are occurring with New Zealand’s cattle industry via the Livestock Sector
Biosecurity Council on developments and lessons from the US situation, and on-farm
biosecurity controls that would help mitigate the risks to livestock in New Zealand.
Official
43. On 8 May, WOAH advised that initial investigations have shown that raw milk from
infected cows is a high-risk material, stating that only milk produced by healthy cows
the
should be used commercially. There is evidence of virus transmission from infected
lactating cows to other animals including cows, cats and poultry. No specific
adaptation of the virus to either humans or mammals has been identified. Several
studies are being carried out to further explore the pathogenesis and transmission
routes of these viruses, including among cattle and from cattle to other animals.
under
44. According to the New Zealand Food Safety Science and Research Centre, it is
important to note that if HPAI virus gets to New Zealand meat and milk from infected
animals would still be safe to consume, provided the meat is properly cooked, and the
milk is pasteurised. The presence of the virus would be a further reason why the
drinking of raw milk is not advised, as HPAI would add to the range of bacterial and
viral pathogens that we already know can be present in raw milk.
45. New Zealand Food Safety has undertaken a food safety risk assessment for the New
Released
Zealand context. The conclusions align with the current international thinking that the
foodborne risk to human health from HPAI is negligible (commercial foods) to low
(non-commercial). Under section 85 of the Animal Products Act, the Director-General
has a power to recall animal products for the purpose of examining, reclassifying,
Page 7 of 16
B24-0348
rectifying or disposal of animal product that is not fit for intended purpose or whose
fitness is in doubt.
46. In the US, pasteurised milk from cattle infected with HPAI has tested PCR-positive for
HPAI genetic material. The Food and Drug Administration has been doing follow-up
testing and reports “
New preliminary results of egg inoculation tests on a second set of
201 quantitative PCR-positive retail dairy samples…show that pasteurisation is
effective in inactivating HPAI.”1
It is likely that the PCR testing is detecting fragments
of viral DNA, but there is no live active virus capable of causing infection. Current
1982
evidence supports pasteurised dairy products being safe to consume.
47. Cat infections with HPAI have been reported in the US, Poland, South Korea, and
Act
France. These cats demonstrated varying degrees of clinical symptoms, including
respiratory and neurological signs, and some mortality. Infection is thought to have
occurred via exposure to infected birds, eating raw poultry meat or, in the US, drinking
of raw milk. There is currently no evidence to suggest cat-to-cat transmission is
occurring.
48. Pigs are known to be infected by Influenza A viruses, however infection of the current
H5N1 strain in pigs has not been widely reported. Current scientific evidence shows
that pigs have a low susceptibility to avian-derived strains of HPAI (that is; virus
isolated from chickens and given to pigs for testing).
Information
49. When pigs have been tested with the mink-derived strains that have mammalian
adaptations present, replication of the virus can be found. The virus is mainly found in
the lower respiratory tract, inducing acute pneumonia. In these studies, no disease-
induced mortality has been observed. The key finding of these studies is that pig-to-
pig transmission was not occurring, likely due to very low virus shedding through nasal
secretions. This implies that further mammalian adaption would be required for pigs to
Official
become a serious HPAI disease vector.
the
Surveillance
50. There are several streams of surveillance underway aimed at detecting HPAI should it
arrive in New Zealand or the Ross Sea region of Antarctica. These include:
a)
a passive surveillance stream complemented by the notifications through
under
Biosecurity New Zealand’s Exotic Pest and Disease hotline. Industry and private
veterinarians have received updated information on recognising HPAI in birds
and spill-over events to mammals;
b)
DOC staff in the sub-Antarctic islands and Antarctic New Zealand staff at Scott
Base have been issued sampling kits and detailed training and instructions on
how to collect samples for HPAI and submit them to the MPI Animal Health
Laboratory;
c)
surveillance of migratory birds and shorebirds not displaying clinical signs of
Released
H5N1 Avian Influzena is underway via a contract between MPI and Dunedin
Wildlife Hospital. This began in September 2023;
1 Updates on Highly Pathogenic Avian Influenza, 1 May 2024, US Food and Drug Administration
Page 8 of 16
B24-0348
d)
Dunedin and other wildlife hospitals will continue to report birds with suspected
avian influenza symptoms via the 0800 number;
e)
study of the ecology and evolution of Low Pathogenicity Avian Influenza viruses
(LPAI) viruses circulating in New Zealand, run by MPI’s Animal Health
Laboratory, involving the sampling and testing of mallard ducks in conjunction
with a Fish and Game New Zealand annual banding programme to track duck
numbers; and
f)
poultry export testing for avian influenza to meet OMAR and enable export of
day-old chicks and hatching eggs. Managed by the poultry industry, with lab
1982
results being provided to MPI. All non-negative samples are sent to MPI Animal
Health Laboratory for confirmatory testing.
Act
51. The Ministry of Health is working with the Institute of Environmental Science and
Research (ESR) to explore opportunities to further enhance surveillance for avian
influenza and to consider avenues for improvement. HPAI is currently a notifiable
disease in New Zealand. As such any case must be reported to the local Medical
Officer of Health at the National Public Health Service. ESR coordinates surveillance
for this.
52. Surveillance to be initiated in the event of a HPAI incursion in New Zealand is outlined
in the section that follows around our response approach.
Information
Legal framework
53. MPI is considering the legislative tools to use to manage HPAI. The primary piece of
legislation for response will be the Biosecurity Act 1993, in conjunction with the Animal
Products Act 1999 and the Food Act 1999 as necessary to manage any risks
Official
associated with consuming animal products.
54. There are a range of powers under the Biosecurity Act that can be used to manage
HPAI. Where the use of these powers directly results in a person experiencing losses,
the
this is likely to entitle the person to compensation under the Act.
55. MPI is considering if there are tools under the Biosecurity Act that would enable
effective management of an incursion in poultry operations 9(2)(g)(i)
. This is appropriate, given that establishment in wildlife
under
has been a hallmark of incursions overseas.
HPAI response components
56. While some wild birds arrive in New Zealand year-round, the next high-risk window for
the arrival of HPAI is believed to begin in September when migratory birds return from
the northern hemisphere. The risk continues throughout Spring/Summer. Over the
coming months, we will continue to work with our One Health Partners and the
Released
industry to finalise those planning and preparedness aspects that need to be resolved
and/or enhanced.
Page 9 of 16
B24-0348
57. We aim to rapidly detect an incursion into New Zealand, contain further spread where
possible and implement effective disease control operations. The plan includes:
a)
surveillance – maintaining (and expanding as needed) surveillance to be able to
detect any arrival of HPAI in New Zealand (including offshore sub-Antarctic
Islands, and also in the Ross Sea area of Antarctica) quickly; and surveillance
that will be activated in the event of an incursion to find additional infected
places;
b)
industry - work will continue with industry to move toward better biosecurity
practices at the farm and shed level. These practices that prevent contact
1982
between wild birds and poultry can help prevent farms from becoming infected.
This includes options for free-range farms (such as housing to limit contact
between commercial and wild birds);
Act
c)
trade and market access work will continue (to implement compartment
approach 6(a)
;
d)
if HPAI is detected in commercial poultry, we will enhance surveillance in other
commercial poultry and restrict movements off infected and suspect properties.
Such a finding will also require increased surveillance in wild birds around the
locality of the infected place. Infected places will require depopulation, with
disposal of culled chickens and other infectious material like chicken litter -
operational planning for these activities is underway. Although the government’s
Information
role in depopulation and disposal (especially over the medium-to long-term) is to
be determined, this is an activity that must be completed to prevent further
spread and limit spill over events;
e)
our plans do not include emergency vaccination of poultry. However, should
industry wish to vaccinate commercial flocks over the longer term, MPI will assist
with the approval process as well as ensuring that vaccination plans take
account of the level of surveillance required in vaccinated flocks to ensure that
Official
the vaccine has adequate coverage of the circulating strain; and
f)
wildlife - if HPAI is detected in wild birds, we will initiate additional surveillance in
wild birds and commercial poultry (as above). The DOC vaccination trial on
the
captive endangered species is well underway, and the plan and approach to
manage these wild birds and vaccinate additional captive populations in the
event of an incursion is to be finalised by DOC. No movement controls or
depopulation would be implemented for wild birds. Disposal options for large-
scale mortalities of wildlife are yet to be worked through.
under
g)
Public health – MOH is developing its plans for public health messaging and
managing the risks for people that could be exposed to HPAI through contact
with dead or sick animals.
58. MPI has a draft operational plan to describe procedures for farm-level response
actions (including movement controls, destruction, disposal, cleaning and disinfection).
This plan will be used in an MPI-led response, but the operational procedures could
also be used by industry operators to manage activities around disposal and cleaning
Released
themselves.
59. The sections that follow describe in more detail the procedures and planning
supporting each of these components.
Page 10 of 16
B24-0348
60. If MPI were required to mount an operational response to an incursion of HPAI, we
would use the service delivery capabilities of biosecurity panel providers to respond
(MPI has an existing procurement panel of providers in place to support responses).
Response surveillance and tracing
61. Following initial detection, surveillance aims to rapidly detect all cases of HPAI in
poultry, to understand the extent of HPAI spread in poultry and wildlife.
1982
62. The aim of tracing following the initial HPAI detection is to backwards and forwards
trace the index case (that is, the first identified case) to determine the source of
infection and to identify where the disease may have spread to. This allows further
Act
spread to be curtailed by identifying possibly infected locations and stopping those
from moving risk goods or animals any further. This activity stops the network of
infected places from growing.
63. Tracing allows the impact on the poultry sector to be reduced by following up potential
disease spread events caused by human activities. Understanding the network of
disease spread provides information that supports the maintenance of supply of
poultry and products to both the domestic and overseas markets, where possible.
64. Surveillance planning for a response is in place and tracing plans are being
Information
developed.
Movement control
65. The objective of movement controls is to prevent the spread of HPAI through
controlling the movement of commercial poultry species and associated risk goods
that can be prevented from moving. Ongoing movement controls are an important
Official
measure for those countries where the large scale of poultry production and/or exports
makes national control programmes economically justifiable. Movement controls will
not be implemented in free-living (wild avian) populations as this is infeasible based on
the
international experience. The use of wildlife risk zones has proved successful for
communicating with poultry owners.
66. Details of any imposed movement controls will be circulated through different media
and social media channels supported by a clear communication plan. International
under
counterparts have used movement control zones of various sizes depending on the
characteristics of a geographic region, local wild bird populations, and the density of
poultry premises in the region.
67. Our operational plan proposes different zones to be placed around infected properties,
in line with overseas experience and planning. The UK implements a three-kilometre
zone around an infected place with a ten-kilometre surveillance zone surrounding that.
The New Zealand approach to movement control zones for commercial poultry is
being worked on including financial and operational considerations. In the event of a
Released
detection in wildlife, we will implement a wildlife risk zone that will help to
communicate to poultry owners in the vicinity that they need to implement greater
focus on biosecurity at the farm level to prevent contact with wild birds.
Page 11 of 16
B24-0348
Depopulation
68. The objective of depopulation is to reduce disease spread while ensuring animals are
killed humanely, as required by section 12 of the Animal Welfare Act. The process
should aim to be completed within 48 hours of notification of the infection.
69. The methods used to depopulate domestic and commercial poultry bird populations
will be dependent on type of farming system, availability of resources, social license,
human safety, and cost.
1982
70. Inhalation of CO2 gas is recognised as the most feasible, acceptable and effective
depopulation method for large numbers of birds. Other methods such as foaming, and
Act
mechanical methods could be considered at smaller scale. Ventilation shutdown is not
recommended for animal welfare reasons but could be considered in an emergency.
Disposal
71. The objective is that disposal of infected carcasses, litter, materials, and equipment is
conducted in a biosecure manner as quickly as possible after depopulation operations
are completed, to reduce any further spread of the virus. Disposal operations are
conducted by trained individuals following all biosecurity, health and safety,
environmental, and hygiene protocols.
Information
72. Disposal can be either on or off the Infected Place. The method will be determined by
risk assessment, considering factors such as the availability of an appropriate on-site
location that meets the criteria for biosecure disposal, proximity to an appropriate off-
site disposal location, and the risk of spread through transportation. Any impact on
businesses and the wider community must also be considered.
Official
73. The most likely on-site disposal methods include composting and deep burial. If on-
site disposal is deemed not suitable or practical then off-site disposal methods could
be used including deep burial at a landfill facility, rendering, or mass disposal at a
the
centralised site by deep burial, incineration, or composting.
74. The poultry industry has some existing ability and technical expertise to manage
disposal within their industry with oversight, support, and guidance from MPI. Other
sectors have existing disposal capability through service providers engaged to carry
under
out business-as-usual disposal operations. These existing relationships could be
utilised with oversight, support, and guidance to strengthen biosecurity from MPI.
75. Our planning on this has drawn from the operational plan developed for Foot and
Mouth Disease (FMD), which has explored options and processes around deep burial,
composting and biosecure transport if offsite disposal is needed.
76. Disposal in the wildlife sector may require One Health partners to ensure logistics and
equipment are available if required. This needs to involve local and regional councils
Released
and is being worked on.
Page 12 of 16
B24-0348
Decontamination
77. Decontamination is designed to reduce the virus load below infective levels and
applies post-depopulation, which requires a combination of cleaning, disinfection and
a stand-down period. This is applicable across the commercial poultry sector,
backyard poultry and zoos/wildlife parks.
78. There is a cleaning and disinfection process as part of the normal commercial industry
production cycles. This varies between the different companies and production sites. 1982
Some companies have processes that would provide effective decontamination for
this virus, particularly the export sector breeder facilities and hatcheries. A detailed
cleaning process would be limited in smaller and backyard style production sites.
Act
79. After decontamination is completed, a minimum of a 28-day stand-down period must
occur before restocking. If decontamination/disinfection cannot be performed
effectively to a level of assurance, then place-based stand-down periods and fallowing
are the only possible options. These standdown periods could have significant impacts
on the industry, as these can range for periods of 120 days (or more).
80. MPI’s operational planning for cleaning and disinfection is based on our detailed
planning in place for other contagious viral pathogens such as FMD and will include
removal of all organic material and application of appropriate agents to kill the virus.
Information
81. It is not practical to undertake decontamination of the environment for wildlife
outbreaks. Wildlife hospitals and conservation facilities can incorporate
decontamination into their preparedness and contingency plans with the appropriate
guidance and standards.
Official
Vaccination
82. Emergency vaccination of poultry is not recommended as part of New Zealand’s
the
approach to an incursion of the currently circulating strain of HPAI. While vaccination
may play a future role in industry adaption2 to living with H5N1 establishing in New
Zealand wildlife, the use of vaccination in commercial poultry has significant
operational consequences. These include high levels of surveillance to monitor
circulating strains of disease and the need to update vaccines to ensure adequate
suppression of spread.
under
Communications and stakeholder engagement
83. Communications material is being produced between One Health agencies, to ensure
consistency of messaging. Material is also being produced for specific stakeholder
groups that may be at risk (for example; duck hunters, those engaging in cultural
muttonbird harvest).
Released
2 Internationally, vaccination is regarded as providing limited benefit for meat chickens due to their short
lifespan (typically around six weeks), but is of increasing interest for layer hens, which often produce for over
two years.
Page 13 of 16
B24-0348
84. Regular meetings between MPI officials and industry have been occurring, both at the
senior and technical level. This includes regular engagement with the industry bodies
such as the Poultry Industry Association of New Zealand (PIANZ), the Egg Producers
Federation (EPF), and New Zealand Feed Manufacturers Association (NZFMA).
These meetings are being utilised to explore if industry resilience and biosecurity
preparedness is sufficient for any potential incursions.
85. MPI has also been engaging with the livestock industry, Fish and Game, and other
groups/organisations as appropriate.
1982
86. A High-Level Advisory Group composed of a recognisable and well-regarded
international expert from each of the three One Health domains has been formed.
Act
They have been convened to get their advice about response approaches and their
input about international developments. We would expect this group to be of
significant value to senior decision-makers in the event of an incursion.
One Health partner activities
Department of Conservation
87. Vaccination can be used to reduce susceptibility to infection. In addition, should a
vaccinated animal become infected, clinical signs and shedding of the virus are
Information
reduced.
88. DOC, supported by MPI, is carrying out a vaccine safety and efficacy trial on five
endangered captive wild bird species (ten individuals of each species, 50 birds in
total). The vaccine used by DOC is one of the two vaccines registered by MPI for use
in New Zealand and held in a DOC-maintained vaccine bank.
Official
89. The trial began in late January 2024, and all birds have received both of the required
vaccination doses. The species in the trial are takahē, red-crowned kākāriki (as a
proxy for kākāriki karaka), tūturuatu, kakī and kākāpō. No adverse events have been
the
observed in any birds. Preliminary results from the vaccine efficacy testing are
promising, indicating takahē and tūturuatu are producing antibodies for HPAI. Further
results are expected over the next few months.
90. There are currently no plans to allow use of vaccination for any other wild birds at this
under
stage, outside of specific New Zealand taonga species where other protective
measures are unavailable and the potential impact of the loss of individuals to
conservation programmes is severe. Capturing and vaccinating free-living birds is
unlikely to be viable on anything other than a very small scale. DOC is working on a
plan beyond the trial as to their approach for protecting these highly endangered
captive species.
91. DOC preparedness for HPAI is focused on making sure Districts are ready to respond
to outbreaks and supporting engagement with mana whenua and impacted
Released
stakeholders (for instance, councils, volunteer groups, DOC permitted operators).
Page 14 of 16
B24-0348
Ministry of Health / Health New Zealand
92. The operational response (including rapid detection and containment) to a human
outbreak of H5N1 avian influenza will be managed by the National Public Health
Service in Health New Zealand. The
New Zealand Influenza Pandemic Plan and the
Communicable Disease Manual contains the Government's plans for this scenario and
are both being updated in light of the risk of an avian influenza outbreak.
93. A joint MOH and Health NZ Incursion Response Plan is being drafted. This plan aims 1982
to:
a)
outline the strategic and operational response to HPAI arriving in New Zealand in
animals, with the aim of managing risks to humans;
Act
b)
inform the health contribution to interagency response; and
c)
manage information needs from the public, media, and stakeholder
organisations, particularly those that employ workers who may be at increased
risk.
94. The Ministry of Health and Health New Zealand, with input from WorkSafe, are
proactively developing more detailed advice for the public to minimise exposure and
risk of infection, especially for people undertaking specific occupational risk activities
to be communicated as the risk profile changes. Information
95. MOH is closely monitoring the literature on genetic and antigenic changes in A(H5N1)
and its various genotypes, particularly from human cases.
Next steps
Official
96. Officials would value the opportunity to speak further with you regarding this briefing to
clarify next steps and any questions you might have.
the
97. We will continue to provide you with regular updates on the progress with our
planning, and the various components of this as outlined in this briefing.
under
Released
Page 15 of 16
B24-0348
Recommendations
98. It is recommended that you:
a)
Note that
MPI will continue to provide regular updates on the progress with our
planning, and the various components of this as outlined in this briefing.
1982
NOTED
b)
Note that a paper for you to take to Cabinet will be prepared, outlining Act
decisions that can be made in advance of an incursion.
NOTED
c)
Agree to forward this Briefing to the Minister of Health, Minister of
Conservation, Minister for Trade and Minister for Hunting and Fishing
YES / NO
Information
Official
Stuart Anderson
Hon Andrew Hoggard
the
Deputy Director-General
Minister for Biosecurity
Biosecurity New Zealand
/ / 2024
under
Released
Page 16 of 16
B24-0348
6(a)
1982
Act
Information
Official
the
under
Released
Page 1 of 1
Appendix One

1982
Act
Information
Official
the
under
Released
HPAI background
1. In 2024, you received an aide memoire covering industry issues for an initial meeting
with poultry industry leaders (AM24-0041
Meeting with Poultry Industry Association
New Zealand, Egg Producers Federation New Zealand and New Zealand Feed
Manufacturers in January) and two briefings about HPAI (B24-0014
High Pathogenicity
Avian Influenza in April and B24-0348
Preparing for High Pathogenicity Avian
Influenza in May).
1982
2.
This aide memoire will briefly recount key points but will not attempt to repeat this
background material in detail.
Act
3.
Avian influenza or ‘bird flu’ refers to a disease caused by influenza Type A viruses.
These spread naturally among wild birds, particularly waterfowl, and can infect a wide
range of bird species, and sometimes cross over into infecting mammals.
4.
A strain of HPAI has emerged and spread across the northern hemisphere and the
Americas. This strain has now reached the Antarctic Peninsula.
5.
Unlike many biosecurity threats, HPAI is not expected to be brought to New Zealand
by human activity, but rather by wild birds. For this reason, it is not likely that it can be
kept out of New Zealand in the long term, or eradicated if it establishes in the wild bird
Information
population.
6.
This strain of HPAI has potentially significant impacts on the poultry sector, wildlife
and biodiversity, and public health. MPI, the Department of Conservation (DOC) and
the Ministry of Health (MOH) are working together in a ‘One Health’ approach to
develop response options to protect our native species, mitigate the impact on the
poultry sector and protect human health.
Official
7.
This paper will focus on impacts on the poultry sector, and how MPI and the sector
can prepare for a HPAI incursion.
the
Recent Australian developments
8.
In May 2024 avian influenza was detected at a poultry farm in Victoria, Australia.
Preliminary testing showed it was not the same strain as the one currently spreading
under
worldwide, and is likely to have emerged as a local mutation from a Low Pathogenicity
Avian Influenza (LPAI) strain. MPI is engaging with Australian counterparts to follow
the progress of their investigation.
9.
The Department of Health Victoria recently advised of a human case of HPAI (H5N1).
A young child who had recently returned from India tested positive for HPAI in early
March 2024 – this infection was acquired outside of Australia and MOH considers it
does not change the overall risk to humans in New Zealand, which remains low.
Released
AM24-0479
Page 2 of 8
Significance of poultry industry
10. Figures from 2022 show that New Zealand produced around 120 million chickens from
151 farms, largely for the domestic market. The value of the chicken meat industry to
New Zealand in 2022 was roughly $1.8 billion, with international trade accounting for
only a small percentage of this figure.
11. Chicken is New Zealand’s most widely consumed meat at an average of just over
40kg per person per year. It is roughly equivalent to the combined per capita
1982
consumption of beef, lamb, and pork, and is particularly heavily consumed in low-
income households. An increase in chicken prices would therefore have an impact on
the cost of living.
Act
12. In 2022, there were around 130 commercial egg farms, producing over 90 million
dozen eggs1 per year. In late 2022, industry reported $286 million in annual retail
sales of eggs, with production types being approximately one third each for colony
cages, barn-laid and free-range. This change towards free-range has been driven by
changes in consumer and supermarket expectations as well as the updated
regulations. The regulations were introduced in 2012 through a Code of Welfare
(Layer Hens), which required a decade-long phase out of battery cages for all layer
hens with full prohibition by 2022.
Information
13. Free range producers who do not have the capacity to house all their birds inside are
the industry group at highest risk from HPAI, as it is extremely difficult to protect their
flocks from contact with wild birds or their faeces.
14. The total value of poultry meat and meat products, and egg and egg product, exported
from New Zealand in 2022 was $138.4 million. In 2024, this is expected to be
approximately $175 million. Poultry meat and meat products contribute approximately
Official
46 percent of the value of poultry exports, with the export of genetic material in the
form of fertile eggs or day-old chickens contributing around 42 percent. Eggs and egg
products make up approximately 12 percent of the value of exports.
the
15. In 2023, New Zealand exported 3.2 million day-old chicks and 6.2 million
fertile/hatching eggs. This market has developed because of the high health status of
New Zealand poultry. Primary markets are South East Asia (including Indonesia,
Philippines, Malaysia, Thailand, and China) and the Pacific (Solomon Islands, Fiji,
under
French Polynesia). These markets are particularly sensitive to the health status of
New Zealand poultry.
Government-Industry Agreements (GIA)
16. The Poultry Industry Association of New Zealand (PIANZ) were approved on 11 April
2023 to represent the poultry meat sector (commercial processing of chicken, turkey,
duck, quail, and geese) of New Zealand under the Government Industry Agreement
(GIA) for the purposes of Part 5A of the Biosecurity Act 1993 (the Act). This was
Released
confirmed on 21 June 2023 when PIANZ signed the GIA deed in the Minister for
Biosecurity’s office.
1 Egg industry production statistics are normally reported in dozens – 90 million dozen comes to around a
billion eggs.
AM24-0479
Page 3 of 8
17. The GIA Deed outlines the principles for the partnership and the commitments that
each signatory makes to engage in the wider biosecurity system. This includes co-
investment to improve the collective biosecurity capacity and capability of industry and
government in readiness and response.
18. The layer hen industry, represented by the Egg Producers Federation of New Zealand
(EPF), is yet to sign the GIA at this time.
1982
Preparedness activities
19. MPI and partner agencies DOC and MOH have multiple workstreams aimed at
Act
improving New Zealand’s preparedness for an HPAI incursion. These include:
a)
DOC staff visiting the sub-Antarctic islands and Antarctic New Zealand staff at
Scott Base have been issued sampling kits and given training in their use;
b)
surveillance of migratory birds and shorebirds not displaying clinical signs of
HPAI is underway via a contract between MPI and Dunedin Wildlife Hospital;
c)
Dunedin and other wildlife hospitals will continue to report birds with suspected
avian influenza symptoms via the 0800 number;
d)
study underway of the ecology and evolution of Low Pathogenicity Avian
Influenza viruses (LPAI) viruses circulating in New Zealand, run by MPI’s Animal
Information
Health Laboratory, in conjunction with Fish and Game New Zealand;
e)
additional awareness material provided to practicing veterinarians;
f)
an app for recording information on suspect cases currently undergoing field
trials; and
g)
a webpage with HPAI information has ‘gone live’ on the MPI website2, and some
of this material will be printed in leaflet form for distribution to the appropriate
Official
target audiences.
20. DOC has a vaccine trial underway on five endangered native bird species, and is
the
focused on making sure districts are ready to respond to outbreaks and supporting
engagement with mana whenua and impacted stakeholders (for example, councils,
volunteer groups, DOC-permitted operators).
21. MOH is updating the New Zealand Influenza Pandemic Plan and the Communicable
under
Disease Manual containing the Government's plans in light of the risk of an avian
influenza outbreak.
22. MOH and Health New Zealand, with input from WorkSafe, are developing detailed
advice for the public to minimise exposure and risk of infection, especially for people
undertaking specific occupational risk activities.
Released
2 https://www.mpi.govt.nz/biosecurity/pests-and-diseases-not-in-new-zealand/animal-diseases-not-in-nz/high-
pathogenicity-avian-influenza-and-the-risk-to-nz/
AM24-0479
Page 4 of 8
Risk factors for infection
23. An American industry publication3 outlined a list of risk factors that is likely to be
broadly applicable to New Zealand if HPAI becomes established in wild birds:
a)
longer lived poultry are at higher risk, including breeders, turkeys and table-egg
laying chickens. Short-lived meat chickens are at lower risk;
b)
farms with more poultry houses are at higher risk of virus introduction. The larger
the farm area, the greater the chance wild birds will deposit virus on the farm; 1982
c)
farms with more birds per house are at higher risk due to increased entry
frequency of personnel and equipment.
d)
poultry requiring personnel and equipment to enter houses more frequently are
Act
at higher risk. More trips (for example, harvesting birds in multiple ‘cuts’) in and
out of the house means more risk of HPAI introduction.
e)
poultry exposed to natural ventilation (the highest risk) or high-velocity ventilation
systems are at higher risk. Unscreened inlets and entrances increase potential
exposure.
f)
farms with high labour requirements are at higher risk due to the increased risk
of personnel turnover. This requires increased attention to training and the
designing of biosecurity procedures to be as foolproof as possible.
g)
farms attracting feral birds are at higher risk, including those in migratory flyways,
near standing water, with attractive nesting and resting sites or other attractants.
Information
h)
turkeys are at higher risk since a lower dose of virus is required to infect them.
Additional biosecurity precautions
24. A range of biosecurity precautions can be imposed, mirroring the risk factors above.
These include the following:
Official
a)
identifying which facilities are at highest risk, and planning biosecurity measures
accordingly.
the
b)
reducing the need for people and equipment to enter houses by utilizing remote
monitoring, automated equipment and robotics as much as possible;
c)
using foot baths and changes-of-clothing when moving between sheds;
d)
designing farms and houses to eliminate feral bird and animal attractants and
maintain them properly;
under
e)
keeping feed and water free of contamination from wild birds;
f)
designing ventilation systems to accommodate biofilters and reducing aerosol
dispersion;
g)
regular washing and disinfection of hard surfaces in each shed;
h)
providing adequate training and supervision for managers and staff so they fully
understand biosecurity measures and the need to implement them;
i)
designing or modifying houses to create entrances for only clean personnel and
equipment, and exits for potentially contaminated personnel and equipment, to
Released
reduce the potential for cross contamination and the chances of operational
biosecurity errors; and
3 Gondor, E.
Rethinking Poultry Farm Biosecurity in Response to HPAI. WattPoultry, Feb 2023.
AM24-0479
Page 5 of 8

1982
Act
Information
Official
the
under
Released

1982
Act
Information
Official
the
under
Released

1982
Act
Information
Official
the
under
Released
Appendix One: Industry Participants
9(2)(a)
1982
Act
Information
Official
the
under
Released
AM24-0479
Page 1 of 1
Appendix One
 Priority
Priority – Low
Security Level – In Confidence
To:
Hon Andrew Hoggard, Minister for Biosecurity
From: Julie Collins, Deputy Director-General Policy & Trade
1982
Biosecurity Portfolio Priorities
Act
Date 11 June 2024
Reference
AM24-0528
Purpose
•
This aide-memoire provides you with information about Biosecurity portfolio priorities
for discussion.
Biosecurity Portfolio Priorities
Out of scope
Information
Official
3.
Attached as
Appendix One is an A3 that outlines Biosecurity portfolio priorities.
These include your agreed priorities and other areas critical to achieving better
biosecurity outcomes for New Zealand.
the
Out of scope
under
Minister / Minister’s Office
Released
Seen / Referred
/ / 2024
AM24-0528
Page 1 of 1

1982
Act
Information
Official
the
under
Released
 Priority
Priority – Low
Security Level – In Confidence
To:
Hon Andrew Hoggard, Minister for Biosecurity
From: Stuart Anderson, Deputy Director-General Biosecurity New Zealand
1982
Update on Highly Pathogenic Avian Influenza
Act
Date 4 July 2024
Reference
AM24-0655
Purpose
•
This aide-memoire provides an update on Highly Pathogenic Avian Influenza (HPAI) in
Australia, avian influenza surveillance in New Zealand and environmental persistence
of avian influenza.
Australia Update
Information
1.
On 19 June 2024, a poultry farm in the Hawkesbury region in New South Wales
(NSW) was confirmed positive with HPAI, strain H7N8. A second poultry property was
confirmed with the same strain on 22 June, about 1.5 kilometres away from the initial
infected property.
2.
On 27 June 2024, a poultry farm in Canberra was confirmed with the H7N8 strain. The
Official
route of transmission has been linked to movement of eggs and associated materials
from one of the infected properties in NSW. With confirmation of an H7 strain in
Canberra, Australia is still free from the H5N1 highly pathogenic strain of concern.
the
3.
This brings the total number of infected farms in Australia to 11, with eight cases in
Victoria (H7N3 and H7N9), two cases in NSW (H7N8), and one case in Canberra
(H7N8). Reports from Australia indicate these detections relate to three separate
spillover events. Restricted areas have been placed around all sites, and
depopulation, cleaning and disinfection of all sites is underway.
under
4.
While it isn’t clear exactly why Australia has experienced these isolated outbreaks of
HPAI, research indicates that climate may be a factor in Australia’s outbreaks.
Australia is more prone to big flooding events that cause a large expansion of
waterfowl numbers that are naïve to disease, leading to circulation of viruses, followed
by extended dry periods that cause wild birds to interact more with poultry in an
attempt to find food and water.
Released
AM24-0655
Page 1 of 3
5.
Experts have advised that differences in New Zealand’s climate, along with a smaller
poultry population, and greater geographic isolation with fewer birds arriving from
places with high rates of Low Pathogenic Avian Influenza (LPAI) circulation, means
that fewer transmission events of all types occur here.
6.
The Ministry for Primary Industries (MPI) is in contact with our counterparts in
Australia, including the Chief Veterinary Officer of Australia, to ensure we capture any
lessons learnt during their response to the outbreaks. Arrangements are underway to
send MPI officials across to Australia to understand the eradication efforts first-hand.
1982
New Zealand Surveillance of Avian Influenza
Act
7.
Since 2004, MPI’s Animal Health Laboratory (AHL), in conjunction with the
Department of Conservation (DOC) and Fish & Game New Zealand, has annually
carried out surveillance targeting non-migratory waterfowl and migratory birds over
summer months. The initial focus was testing migratory birds, however results
indicated that migratory birds posed a very low risk for avian influenza, so the focus
has shifted to resident waterfowl since 2010. These animals are known to be in very
close proximity to migratory birds at mingling sites and are competent hosts of LPAI.
8.
Results show that the same strain of LPAI that has been present for the past 20 years
continues to be detected in mallard ducks, which is closely related to some of the
Information
historic United States strains of LPAI. It appears to have been around since the 1970s
and is relatively stable — there are no mutations of concern.
9.
In mallard ducks, the LPAI prevalence in New Zealand is highly variable depending on
the year, location, and time of year.
10. For the 2024 summer season, AHL has completed the avian influenza PCR screening
Official
for the annual survey of mallard ducks in New Zealand. Four locations were visited –
Parakai Springs, Paeroa, Kaituna, and Te Awamutu. 2,560 samples were collected,
from 1,280 mallard ducks. These were tested for the presence of avian influenza virus.
the
A total of 997 samples from 632 mallards tested positive for avian influenza. All
positive samples were tested using H5- and H7-specific avian influenza PCR, with no
positive results, meaning that all detected positives are LPAI.
Environmental Persistence
under
11. Research shows persistence of avian influenza in the environment is varied, and
depends on the strain studied. Environmental contamination of HPAI in large bird
colonies is high, such as nesting seabirds or waterfowl. This is due to bird faeces
containing large amount of infectious virus. In freezing conditions (-20 degrees
Celsius), it can last up to 12 months, with the ability to survive repeated freeze-thaw
cycles.
12. In fresh and salt water, HPAI has been shown to survive for months in cool
Released
temperatures. However, survival of the virus varies across different temperatures, pH,
and other environmental conditions; such as rainfall and numbers of birds present to
shed virus.
AM24-0655
Page 2 of 3

1982
Act
Information
Official
the
under
Released
 Priority
Priority – Low
Security Level – In Confidence
To:
Hon Andrew Hoggard, Minister for Biosecurity
From: Stuart Anderson, Deputy Director-General Biosecurity New Zealand
1982
Update on Highly Pathogenic Avian Influenza
Act
Date 19 July 2024
Reference
AM24-0678
Purpose
•
This aide-memoire updates you on work to prepare New Zealand for a Highly
Pathogenic Avian Influenza (HPAI) incursion.
Recent International Developments
Australian funding boost for HPAI preparedness
Information
1.
Australia is currently responding to three outbreaks of HPAI in commercial and
domestic poultry. These appear to be locally-derived mutations from H7 low
pathogenicity avian influenza viruses.
2.
On 10 July, the Australian government announced an additional funding boost of
Official
AUD$6.9 million to prepare for H5N1 HPAI, in advance of the spring return of
migratory birds to Australia from countries where H5N1 HPAI is present.
the
3.
The AUD$6.9 million includes:
a)
$2.2 million for a Wildlife Health Australia One Health surveillance initiative;
b)
$1.95 million for Animal Health Australia to build response capability, including
assessing the potential for avian influenza vaccine use in Australia;
c)
$1.1 million to extend a wild bird surveillance programme for another four years;
d)
$800,00 in communications with stakeholders, industry and the general public;
under
e)
$500,000 to support early detection and response capability in wildlife;
f)
$200,000 to study the location, structure, biosecurity practices and movements
within Australia’s commercial poultry sector; and
g)
$70,000 to look at links between H7 avian influenza in wildlife and the current H7
outbreaks in poultry
United States human infections
Released
4.
Health officials have confirmed four (potentially five) new human cases have become
infected with H5N1 HPAI. According to media, they are workers responding to the
avian flu outbreak at a commercial egg layer operation, and that workers did not apply
PPE in accordance with guidelines.
AM24-0678
Page 1 of 4
 Public notifications
Rise in public reports and laboratory submissions
Public notifications
Rise in public reports and laboratory submissions
1982
Act
Information
5.
Since 2021, a global surge in worldwide H5N1 HPAI cases prompted heightened
vigilance in New Zealand. MPI’s Animal Health Laboratory (AHL) has experienced a
Official
doubling in HPAI laboratory submissions each year since 2021. Between 1 January
and 31 May 2024, AHL received and tested 36 HPAI laboratory submissions,
compared to 17 HPAI submissions during the same period in 2023, and a baseline of
the
eight submissions per year before 2020. This uptick reflects successful engagement
channels and heightened awareness among stakeholders and the wider public about
the importance of reporting suspect cases to MPI.
6.
Sample submissions and public reports via the 0800 Exotic Pest and Disease hotline
continue to increase. Many of the reports are of relatively low value, such as reports of
under
individual dead wild birds where there is no epidemiological evidence to suggest the
presence of HPAI. We triage and consider each of these reports as to whether testing
in the laboratory is required.
Industry preparedness
7.
Industry and MPI are working together to provide information to farmers about what a
detection of HPAI would mean for them at the farm level. Knowledge about the
Released
response will help producers plan for a potential incursion and enable planning for
business continuity.
8.
MPI is working with industry on a project to support improving resilience and tightening
biosecurity on commercial poultry farms. There has been good engagement already
AM24-0678
Page 2 of 4
from the industry bodies, and organisations across the broiler, egg, and genetics
sectors.
9.
Initial engagement with larger companies confirms commercial hatcheries, breeders
and exporters of genetic material (hatching eggs and day-old chicks), and producers
of broilers generally have a strong focus on biosecurity and are being proactive at
improving it.
10. The egg production sector has a larger number of independent operators. A key task 1982
for MPI is to improve our understanding of smaller commercial enterprises, particularly
those operating as ‘free range’. Within this sector, biosecurity practices and
awareness of HPAI are highly variable, and many smaller free-range producers are
Act
yet to develop concrete plans of how they would reduce their risk of infection if HPAI
was detected in New Zealand.
HPAI web portal
11. All HPAI material on the MPI website was refreshed in mid-May 2024. One Health
partner agencies, (Department of Conservation, Ministry of Health, Health New
Zealand) have information that refers to and cross-references the MPI website, and
communications teams remain in regular contact across agencies and industry. The
site can be found at: https://www.mpi.govt.nz/biosecurity/pests-and-diseases-not-in-
Information
new-zealand/animal-diseases-not-in-nz/high-pathogenicity-avian-influenza-and-the-
risk-to-nz/
12. The portal provides information targeted to different audiences and areas of concern,
such as HPAI in commercial poultry, HPAI in domestic poultry, and protecting native
wildlife from HPAI. Some of this material has been printed as factsheets and
distributed to specific target audiences.
Official
13. This material is progressively refreshed as new information comes to hand. For
example, a section on “Dairy and other livestock’ was added in response to the spill-
the
over into dairy herds in the USA.
14. Most recently New Zealand Food Safety (NZFS), added a page on Food Safety and
Human Health, to address concerns about consuming poultry, eggs, and dairy
products. Avian influenza viruses are sensitive to heat treatment, so cooked food and
under
pasteurised milk products are safe to consume.
Other preparedness activities
15. Planning is underway for visits by MPI and industry personnel to Australia and the UK
in August to get first-hand information on how those countries have responded to
HPAI. Four MPI staff and eight industry members are expected to travel on the UK
study tour.
Released
16. Industry Chief Executives were briefed on 18 June on the status of operational plan
development, the range of activities underway, and they discussed plans to involve
industry in relevant workshops.
AM24-0678
Page 3 of 4
17. Destruction, disposal and cleaning and disinfection options for commercial poultry are
currently being prioritised in operational planning through shared activities with
industry.
18. Progress is being made on a joint Cabinet paper between the Ministers for Health,
Conservation and Biosecurity. Agencies are currently in the final stages of drafting,
with expected delivery to Ministers in August 2024.
1982
Act
Minister / Minister’s Office
Seen / Referred
/ / 2024
Information
Official
the
under
Released
AM24-0678
Page 4 of 4
 Priority
Priority – High
Security Level – In Confidence
To:
Hon Andrew Hoggard, Minister for Biosecurity
From: Steven Kelly, Manager, Bilateral Relations & Trade
1982
Meeting with Hon Clare Scriven MLC, Minister for Primary
Industries and Regional Development, Government of South
Act
Australia
Date 24 July 2024
Reference
AM24-0721
Purpose
Out of scope
Information
Official
the
under
Released
AM24-0721
Page 1 of 4
Out of scope
1982
Act
Information
Official
the
under
Released
AM24-0721
Page 2 of 4
Out of scope
1982
Act
Information
Official
High pathogenicity avian influenza (HPAI)
the
14. It is possible that, in the course of your conversation with Minister Scriven, she may
want to discuss three recent outbreaks of HPAI in Victoria, New South Wales and the
Australian Capital Territory.
15. These outbreaks appear to be locally-derived mutations from H7 low pathogenicity
under
avian influenza viruses. There are restrictions and conditions on the movement of live
poultry and fertile eggs into South Australia from these states/territory.
16. The likelihood of any of these Australian strains reaching New Zealand is extremely
low, given their lack of ability to disperse via wild birds.
17. To prepare for the possibility of HPAI H5N1 reaching New Zealand from elsewhere in
the world, MPI is working closely with the poultry industry to improve sector biosecurity
preparedness and response planning. An industry led, government supported,
Released
response is considered the most appropriate approach to managing an H5N1
outbreak in poultry. The response objective would be to eliminate the disease from
commercial poultry operations as it arises while adapting management practices to
reduce the risk of becoming infected.
AM24-0721
Page 3 of 4
18. MPI currently have ongoing contact with Australian officials at the Federal level
regarding HPAI preparedness and response. MPI officials will be visiting Victoria the
week of 29 July to take operational learnings from Australia’s response to date.
1982
Minister / Minister’s Office
Act
Seen / Referred
/ / 2024
Information
Official
the
under
Released
AM24-0721
Page 4 of 4
Out of scope
1982
Act
Information
Official
the
under
Released
AM24-0721
Page 1 of 1
Appendix One
Appendix Two: Talking Points
Out of scope
1982
Act
High pathogenicity avian influenza (HPAI)
Information
•
Understand what early lessons have emerged out of the responses.
•
Seek reflections on how prepared for an incursion relevant industries have been.
•
Ask about any early indications of the potential and timeframes for recovery.
Official
•
Explore any emerging supply chain issues and mitigations
.
the
under
Released
AM24-0721
Page 1 of 1
Appendix Two
 Priority
Priority – Low
Security Level – In Confidence
To:
Hon Andrew Hoggard, Minister for Biosecurity
From: Stuart Anderson, Deputy Director-General Biosecurity New Zealand
1982
Highly Pathogenic Avian Influenza Response Preparedness
Act
Date 29 July 2024
Reference
AM24-0728
Purpose
•
This aide-memoire updates you on the current level of Preparedness for a Potential
Highly Pathogenic Avian Influenza (HPAI) Response.
Current level of Preparedness for a Potential Highly Pathogenic Avian Influenza
(HPAI) Response
Information
1.
The Ministry for Primary Industries (MPI) is working alongside One Health Partners
(the Department of Conservation (DOC), the Ministry of Health, and Health
New Zealand) to develop HPAI response options that represent the best approach for
New Zealand to protect our unique native species, mitigate the impact on the poultry
sector and take preventative measures to protect human health.
Official
2.
An operational response plan for the poultry sector has been developed which details
field-based activities to enable disease control (movement control, surveillance,
destruction, disposal, and cleaning and disinfection). This plan is based on previous
the
response planning, industry workshops, and response plans for HPAI from overseas.
This plan could be enacted immediately if required. A detailed work schedule is
guiding ongoing work to enhance the existing operational response plan, in
conjunction with One Health and Industry partners. Our planning documents will be
updated with the findings of the upcoming study trips to Australia (Victoria) and the
United Kingdom taking place over the next 2 weeks.
under
3.
Field services to enact the current plans can be procured rapidly through our panel
providers, and we are also working on options for provision of services such as
transport and carbon dioxide supply with industry, regional councils, and commercial
operators.
4.
DOC is developing response plans and information for its operational teams around
the country in preparation for the arrival of H5N1. It is also currently conducting a
Released
HPAI vaccination trial in collaboration with MPI.
AM24-0728
Page 1 of 3

1982
Act
Information
Official
the
under
Released
11. We will continue to keep you updated on our work regarding readiness planning, the
various sector agreements under development, and on any developments in the
spread and impacts of the disease internationally.
1982
Act
Minister / Minister’s Office
Seen / Referred
/ / 2024
Information
Official
the
under
Released
AM24-0728
Page 3 of 3
 Priority
Priority – Low
Security Level – In Confidence
To:
Hon Andrew Hoggard, Minister for Biosecurity
From: Stuart Anderson, Deputy Director-General Biosecurity New Zealand
1982
High Pathogenicity Avian Influenza Update
Act
Date 13 August 2024
Reference
AM24-0770
Purpose
•
This aide-memoire updates you on work to prepare New Zealand for a H5N1 Highly
Pathogenic Avian Influenza (HPAI) incursion. It also provides a summary of the
possible implications on free-range producers during an incursion.
International Environment
Information
1.
The United Kingdom has updated its risk assessment of the H5N1 situation to
grade 4: evidence of ongoing mammalian transmission and some non-sustained
zoonotic transmission.
2.
Cambodia has reported a ninth human case from H5N1 HPAI. Two of these cases
occurred in late July early August, both of which were hospitalised. All cases had a
Official
history of recent exposure to sick or dead poultry, seven cases were children, one
case was an adult. There was one fatality.
the
3.
The United States of America has not reported any human cases since 25 July 2024.
Spread of H5N1 among US dairy cows is continuing, with infection reported in
178 herds (as of 5 August).
Study Tours of Australia and the United Kingdom
under
4.
Ministry for Primary Industries (MPI) officials returned from the Australian study tour of
the incursion of locally evolved, non-H5N1 HPAI. Agriculture Victoria have deployed
around 600 of their staff to their state and regional coordination centres, and the
eight infected properties. 6(b)(i)
, with an
objective to eradicate the HPAI and to return to normal trade.
5.
The UK study tour was undertaken from 3 to 12 August, including MPI and industry
members to observe and learn from the UK’s HPAI response.
Released
6.
We will provide you with a debrief of the main findings from these study tours.
AM24-0770
Page 1 of 3

1982
Act
Information
Official
the
under
Released
14. Officials are investigating what impact HPAI may have on the supply of poultry meat
and eggs and, if needed, what potential mitigations may be. Overseas experiences
have shown few countries have had a shortage of product. In some instances, there
has been an increase in cost to consumers, particularly for eggs.
15. There is also evidence that consumer confidence in the safety of poultry products
drops during an HPAI outbreak. Messaging to consumers to alleviate a fear-based
response is occurring now, to ensure the public that our supply chains will be robust,
and poultry products will be safe to consume during an outbreak.
1982
Laboratory readiness
Act
16. MPI’s Animal Health Laboratory is focusing on timely investigations for increased
surveillance:
a)
the laboratory is working on the integration of laboratory equipment (PCR
machines) with the Laboratory Information Management System (the system) for
HPAI, as well as the integration of the field sample collection app with the
system. This integration enables automated data transfer, reduces sample
processing time, and minimises human transcription errors; and
b)
the laboratory is using a scenario-based spreadsheet approach to provide the
best estimates at the time for sample numbers to enhance laboratory
preparedness for HPAI. This is required to determine the necessary stocks of
Information
reagents and consumables, ensuring we have adequate supplies on hand (given
potential delays in overseas deliveries at the onset of a response). This is also
critical to inform the laboratory staff structure.
Next HPAI Update
Official
17. You will receive the next update on HPAI in the week of 26 August.
the
under
Minister / Minister’s Office
Seen / Referred
/ / 2024
Released
AM24-0770
Page 3 of 3
 Priority
Priority – Low
Security Level – In Confidence
To:
Hon Andrew Hoggard, Minister for Biosecurity
From: Stuart Anderson, Deputy Director-General Biosecurity
1982
High Pathogenicity Avian Influenza Update
Act
Date 2 September 2024
Reference
AM24-0807
Purpose
•
This aide-memoire updates you on work to prepare New Zealand for a H5N1 Highly
Pathogenic Avian Influenza (HPAI) incursion. It also provides a summary of the
possible implications on free-range producers during an incursion.
International Environment
Information
California cattle infection
1.
Three California dairy herds tested positive for HPAI, with clinical signs beginning 23
August to 25 August. It is not yet clear how these herds became infected – if it was
from another spillover event or is the same HPAI H5N1 variant that’s spread from
Texas. No human cases have been confirmed in relation to this event.
Official
2.
Animal movement is being tracked and evaluated. The affected dairies have been
placed under quarantine on the authority of California Department of Public Health’s
the
State Veterinarian, and enhanced biosecurity measures are in place. Sick cows are
isolated and are being treated at the dairies; and healthy cows have been cleared to
continue shipping milk for pasteurization.
Other international HPAI updates
under
3.
In Australia, no further cases of HPAI have been detected since 24 June. As a result,
housing orders and movement controls are easing.
4.
In Malaysia, ten human cases of H5N1 bird flu have been recorded so far this year,
four since July. One teenage female died. No evidence of human-to-human
transmission has been reported in association with any of these cases.
5.
Europe is starting to see incursions as Autumn approaches. France and Poland both
Released
had incursions mid- to late- August, after having none since January and February,
respectively.
AM24-0807
Page 1 of 5

1982
Act
Information
Official
the
under
Released

1982
Act
Information
Official
the
under
Released

1982
Act
Information
Official
the
under
Released

1982
Act
Information
Official
the
under
Released