
133 Molesworth Street
PO Box 5013
Wellington 6140
New Zealand
T+64 4 496 2000
4 November 2024
s 9(2)(a)
Ref:
H2024051358
Tēnā koe s 9(2)(a)
Response to your request for official information
Thank you for your follow up request under the Official Information Act 1982 (the Act) to the
Ministry of Health – Manatū Hauora (the Ministry) on 10 September 2024. You requested:
Could you please provide me with the "literature review" and "proposed revised
recommendation" mentioned in paragraph 4 of the May 27 memo as being provided to CV
TAG by IMAC? I've inserted a screenshot below for clarity/convenience:
Please find this information in the links below:
• www.tewhatuora.govt.nz/assets/About-us/Who-we-are/Expert-groups/COVID-19-
Vaccine-Technical-Advisory-Group-CV-TAG/Pfizer-COVID-19-vaccine-in-pregnancy.pdf
• www.tewhatuora.govt.nz/assets/About-us/Who-we-are/Expert-groups/COVID-19-
Vaccine-Technical-Advisory-Group-CV-TAG/Decision-to-use-the-Pfizer-mRNA-COVID-
19-vaccine-for-group-three-and-general-population.pdf
Sorry one more thing - apologies for not including this in my email above, but can you
please also provide the meeting minutes for the CV TAG meeting which took place to
discuss the revised recommendation on 25 May 2021? This is also mentioned in the May
27 memo. I also request any correspondence, documents or other information related to
this meeting.
Hopefully sending two emails doesn't confuse things, please note my request above for
the information provided to CV TAG by IMAC (mentioned in paragraph 4 of the May 27
memo) also. I consider the information requested in both of these emails to be relevant to
my initial request.”
Information within scope of your request is itemised in Appendix 1 of this letter and copies of the
documents are enclosed. The table in Appendix 1 outlines the grounds under which I have
decided to withhold information. I have considered the countervailing public interest in release in
making this decision and consider that it does not outweigh the need to withhold at this time.
Please note the information contained in document 5 titled ‘
COVID-19 and COVID-19 vaccines
in pregnancy update’, was based on evidence available at the time that this summary was
produced (21 May 2021). Hyperlinks are included throughout the summary, and in the section

headed ‘Website URLs for hyperlinks. Many of these hyperlinks now auto-redirect the reader to
content that has been updated since the date that this summary was produced.
Please also note that documents 9 and 10 are held by the Ministry but are drafts and do not
necessarily reflect the views of the Ministry nor the CV-TAG meeting held on 25 May 2021.
I trust this information fulfils your request. If you wish to discuss any aspect of your request with
us, including this decision, please feel free to contact the OIA Services Team on:
[email address].
Under section 28(3) of the Act, you have the right to ask the Ombudsman to review any
decisions made under this request. The Ombudsman may be contacted by email at:
[email address] or by calling 0800 802 602.
Please note that this response, with your personal details removed, may be published on the
Manatū Hauora website at: www.health.govt.nz/about-ministry/information-releases/responses-
official-information-act-requests.
Nāku noa, nā
Kristie Carter
Group Manager, Intelligence, Surveillance and Knowledge Public Health Agency | Te Pou Hauora Tūmatanui
Page 2 of 4
%201.pdf please refer to
pages 20-21.
9
25 May 2021
Draft statement on Long-term effects Released in full.
of Pfizer vaccine (appendices to the
agenda)
10 24 May 2021
Strawman Statement
11 N/A
The effect of body mass index and
Refused under section
arm size on intramuscular vaccine
9(2)(b)(ii) of the Act, as its
delivery and immunogenicity
release would likely
unreasonably prejudice the
commercial position of the
person who supplied the
information.
Page 4 of 4





Document 4
Memo
Pfizer COVID-19 Vaccine – Decision to Use
Date:
20 May 2021
To:
COVID-19 Vaccine Technical Advisory Group
From:
Al ison Bennett, Manager, System Enablers, System Strategy and Policy
For your:
Advice
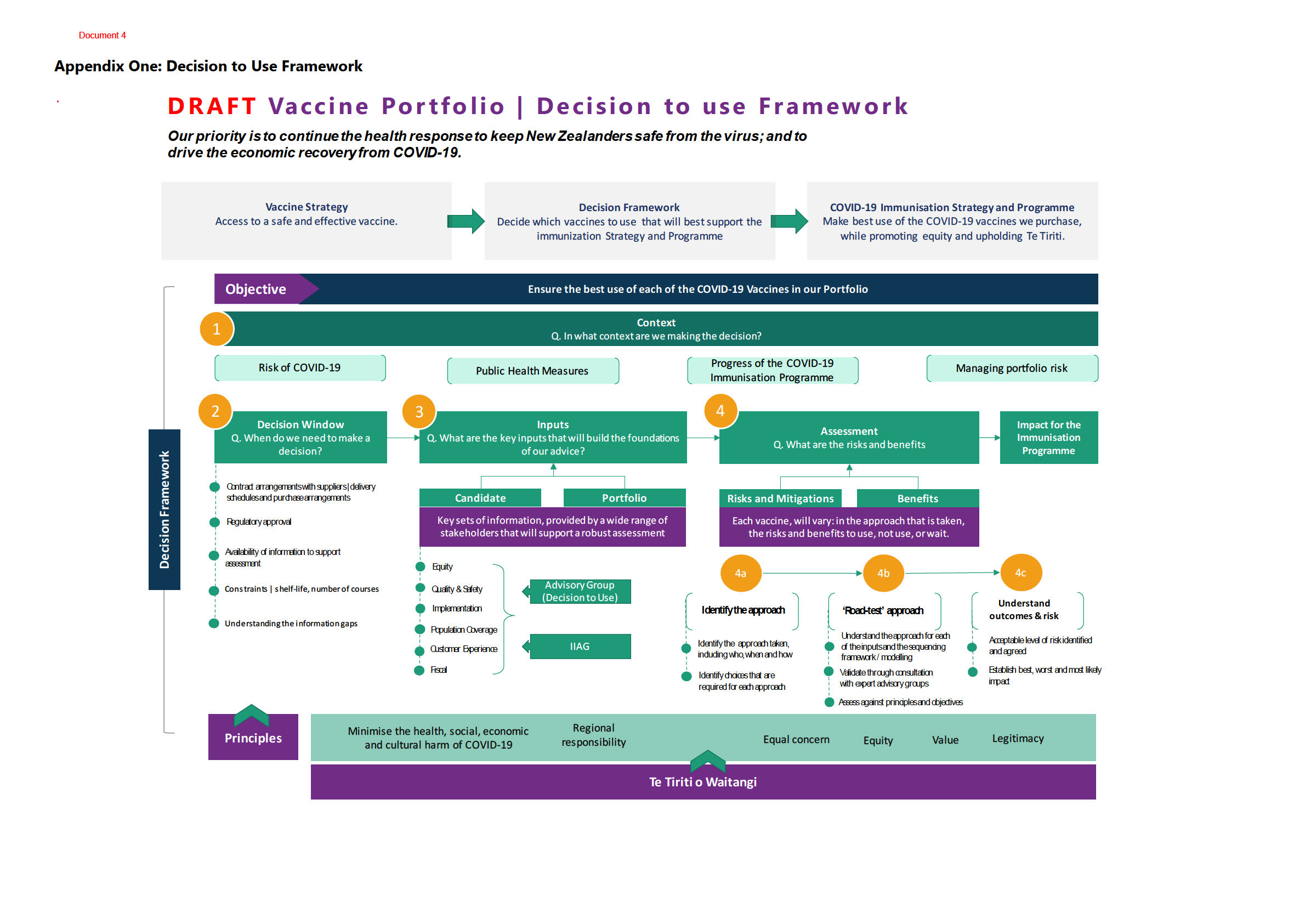
Purpose of report
1.
This paper seeks to confirm if there is additional information available since initial rollout of
the COVID-19 Pfizer vaccine that would impact on proceeding with the group three at scale
ACT 1982
and the general population rol out of the COVID 19 Pfizer vaccine
Background
2.
Advice is provided to Cabinet on each vaccine cand date as it becomes available for use,
without knowing if a future vaccine is going to be more suitable or effective. In order to make
decisions given the uncertainty of uture candidate’s availability, a Decision to Use framework
was developed.
3.
The Decision to Use Framework (please see Appendix One), is centred around the fol owing
four questions for making decisions on how to use COVID-19 vaccines, who to use them for
and when to use them:
INFORMATION
what is the context that the decision is being made in?
when do we need to make a decision?
RELEASED UNDER THE
what are the key pieces of information that wil build the foundations of our advice?
what are he risks and benefits?
Decision to Use - Pfizer COVID-19 Vaccine
4.
Fol owing Medsafe’s provisional approval of the Pfizer mRNA COVID-19 vaccine (Comirnaty,
OFFICIAL
BNT162b2) for people 16 years and over on 3 February 2021, the COVID-19 Vaccine Technical
Advisory Group (CV TAG) met on 4 February 2021 to provide the scientific and technical
assessment of the Pfizer mRNA COVID-19 vaccine, including advice on who is to receive the
vaccine.
5.
The CV TAG completed a scientific and technical assessment of the Pfizer mRNA COVID-19
vaccine, in order to provide recommendations on its use for New Zealand’s Vaccine
Immunisation Programme.
Page 1 of 6

Document 4
6.
Advice was provided to Cabinet which recommended that we proceed with providing access
to the Pfizer COVID-19 vaccine in line with the Sequencing Framework, noting the primary
limitation of use in those 16 years and over, in line with the Medsafe approval.
7.
It was noted that there were no exclusions, or limitations in the advice of the CV TAG that
would material y impact on the implementation of the Sequencing Framework and
Immunisation Programme.
Progressing to group three at scale and into the general population
8.
It was noted that the Ministry of Health would provide further advice on the use of the Pfizer
vaccine, alongside the other vaccines in our portfolio, before we start immunising people
identified in group three at scale (group three includes people in the community most at r sk
of serious il ness and the workforces supporting them).
9.
Given that we are progressing with group three at scale and then moving into the general
population rol out in July, we wish to confirm with the CV-TAG if there is any new
information since initial rollout of the Pfizer COVID-19 vaccine that would materially impact
on the implementation of the Sequencing Framework and Immunisation Programme at this
time. We have attached a summary of the safety data to support your discussion (Appendix
Two)
ACT 1982
10. The initial decision to use Pfizer for groups one and two was made on a basis that there
would be further consideration of other candidat s in our portfolio by the time we were due
to rol out to these groups, however, such regulatory decisions remain pending at this point
in time.
11. Given the ongoing international context supply risks and increasing evidence around COVID-
19 vaccines safety, efficacy and quality and uncertainty around duration, the need for
boosters and managing variants of concern we wil seek CV-TAG advice on managing these
risks in the coming weeks.
INFORMATION
RELEASED UNDER THE
OFFICIAL
Page 2 of 6
 ACT 1982
INFORMATION
RELEASED UNDER THE
OFFICIAL
ACT 1982
INFORMATION
RELEASED UNDER THE
OFFICIAL
 ACT 1982
INFORMATION
RELEASED UNDER THE
OFFICIAL
ACT 1982
INFORMATION
RELEASED UNDER THE
OFFICIAL
 ACT 1982
INFORMATION
RELEASED UNDER THE
OFFICIAL
ACT 1982
INFORMATION
RELEASED UNDER THE
OFFICIAL

Document 4
9(2)(c)
ACT 1982
INFORMATION
RELEASED UNDER THE
OFFICIAL
Page 6 of 6
Document 5
IMAC – not for public distribution.
21 May 2021
Approved for release under the Official Information Act 1982
COVID-19 and COVID-19 vaccines in pregnancy - update.
This is an update of a summary of the evidence around the risks of COVID-19 in pregnancy and the
advice and safety related to giving the vaccine in pregnancy.
COVID-19 disease
In pregnant people
During pregnancy, the immune system is downregulated to prevent an immune response against the
fetal antigens. Particularly affected is the T cell (cellular) immune response that also plays an
important role in the response to viral infection, along with the antibody (B cell) response. It is well
recognised that pregnancy increases the risk of complications from a viral infection, such as
influenza and varicella, and there is good evidence to show that this is also the case for COVID 19.
In pregnancy, COVID-19 carries a substantially increased risk of severe disease requiring intens ve
care unit admission (ICU; over 18 times higher than pregnant women without COVID-19) and
invasive ventilation support and death (over three times higher), although pregnant or recently
pregnant people were not at increased risk of asymptomatic or mild disease(1) Risk of more severe
disease was further increased by older maternal age, higher body mass index and the presence of
pre-existing comorbidities, including diabetes and hypertension.(1)
ACT 1982
A study in the UK found that maternal respiratory compromise due to COVID-19 resulted in
caesarean delivery in almost six out ten of births and one in eight were delivered preterm.(2) In the
US, the rates of preterm birth were nearly ten times higher (45% vs 5.2%) with severe COVID-19
compared with mild disease.(3)
In newborns
For babies of mothers with COVID-19, risk of preterm and neonatal ICU admission is increased, but
not the risk of stillbirth or neonatal death. SARS CoV 2 infection in pregnancy does not appear to
affect fetal growth, adverse neonatal outcomes or the rate of stillbirth.(4) The risk of SARS-CoV-2
infection from the mother to her newborn appears to be smal .(5) However, a systematic review of
SARS-CoV-2 infection in newborns found that, although most were asymptomatic (20%) or had mild
(48%) or moderate (20%) signs of COVID-19. A higher proportion were severely unwell (12%), than
INFORMATION
seen in older children, with dyspnoea the most commonly reported sign.(6)
RELEASED UNDER THE
COVID-19 vaccines
Planning pregnancy
A recent non-peer-reviewed study found both SARS-CoV-2 infection and mRNA vaccine (Comirnaty®)
induced anti-SARS-CoV-2 IgG antibodies were detected in the ovary follicular fluid and the levels
correlated with serum IgG levels. No detrimental effect was seen in follicular function and oocyte
quality biomarkers in patients undergoing oocyte retrieval attending an IVF clinic in Israel.(7)
OFFICIAL
In pregnancy
Although the mRNA COVID-19 vaccines were not specifically tested in pregnant people during their
initial clinical trials, this is always the case, and key vaccines used in pregnancy (influenza and
pertussis) were introduced without specific clinical trials. During preclinical studies in pregnant
animals, no toxicity issues were identified for the mRNA vaccines for COVID-19 or for other vaccine
candidates based on the same platform such as a Zika virus vaccine.(8) There are no specific safety
reasons to exclude pregnant people since, based on how these vaccines work, they are unlikely to
Document 5
IMAC – not for public distribution.
21 May 2021
pose risk to a pregnant person or their unborn child. The mRNA COVID-19 vaccine (Comirnaty) does
not contain live virus and is unable to give someone COVID-19. In addition, the mRNA in the vaccine
cannot enter the nucleus of the cell, it does not interact with human genetic material and breaks
down very quickly by the normal processes after entering the cell.
In most countries, any potential risk from the vaccine is balanced against a substantial risk of
catching COVID-19 and significant risk of illness to the pregnant person and their infant. Risks are
event higher for those with other underlying health conditions. As a result, in recent months more
countries have recommended that COVID-19 vaccines be routinely used in pregnancy.
Ongoing safety surveillance and dedicated registers for pregnancy outcomes are monitoring those
who have received COVID-19 vaccines in pregnancy (see below for latest data) in several countries
There is an international push to get more vaccines developed specifically for use during pregnancy
to protect mother and infant or to be tested during pregnancy.(9) Clinical trials have commenced
testing these vaccines in pregnant and non-pregnant people, including one for the Comi naty®
mRNA vaccine (NCT04754594). One chal enge being faced for this type of clinical trial, in countries
like the US where there is a high incidence of COVID-19, is that it has become unethical to give
placebo and not the vaccine to all the participants (personal communication with Pfizer).
Recent data has shown a protective immune response in people who were vaccinated with mRNA
ACT 1982
COVID-19 vaccines in pregnancy and protective antibodies were transferred to the infant across the
placenta (detected in cord blood at delivery) and in the breastmilk (10, 11) No severe adverse events or
complications in pregnancy or in the newborn were observed.(10) The ntibody levels in pregnant and
lactating participants were equivalent to those who were not pregnant. The immune response to the
vaccine was greater than that to COVID-19 infection.(12) The optimal timing of vaccination is
unknown; maternal and neonatal antibody levels wer directly correlated with time since
vaccination.(11)
In people who are breastfeeding
All vaccines used on the New Zealand immunisation schedule can be given those who are lactating
and breastfeeding. Although there is limited data about the use of the COVID-19 vaccines when
breastfeeding, there is no plausible eason for any safety concerns. No intact spike protein enters
breastmilk from the maternal circulation and free mRNA is destroyed very quickly by enzymes in the
INFORMATION
tissues and blood Any intact lipid nanoparticles present in the breastmilk are destroyed by the
infant’s gastrointestinal tract if ingested.(13)
RELEASED UNDER THE
By giving protection while breastfeeding, immunisation can reduce the risk of the spread of infection
to the baby and k ep the mother well so they can fully look after their baby. Recent data has shown
vaccination while breastfeeding provides protective antibodies for baby in breastmilk.(10, 12, 14)
Those who have delayed receiving COVID-19 vaccine in pregnancy, are highly recommended to
receive it as soon as possible after delivery and do not need to suspend breastfeeding.
OFFICIAL
Safety surveil ance data for COVID-19 vaccines given in pregnancy.
The safety of the mRNA COVID-19 vaccines when given in pregnancy is being monitored globally. The
Centers for Disease Control and Prevention (CDC) in the US published data for 14 December 2020 to
28 February 2021 from the ‘v-safe After Vaccination Heath Checker’ (a smartphone-based reporting
system), v-safe COVID-19 Vaccine Pregnancy Registry (linked to v-safe) and the Vaccine Adverse
Event Reporting System (VAERS; a passive reporting system).(15) A total of 35,691 v-safe participants


Document 5
IMAC – not for public distribution.
21 May 2021
were pregnant at the time of vaccination and just under 4,000 enrolled in the v-safe pregnancy
registry (see below).(15)
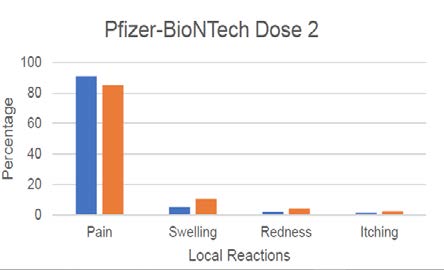
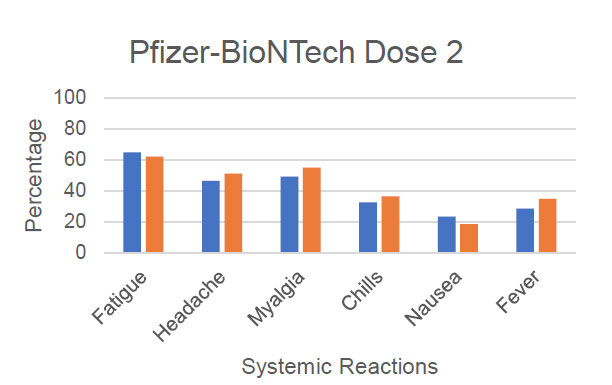
No significant difference were reported to v-safe between local and systemic reactions by pregnant
and non-pregnant after the first or second dose of mRNA vaccine (see Figure 1 for data presented by
author to CDC).(15, 16) As seen across all vaccine recipients, injection site pain was the most reported
Figure 1: V-safe systemic and local reaction reports on day 1 post-vaccination in self-reported
pregnant and non-pregnant women aged 16-54 years (to 13 Jan 2021; presented by
T Shimbukaro, 1 Mar 2021)
ACT 1982
response (70-80%) with increasing incidence of fatigue, heada he, myalgia and chills after dose 2 –
these responses are identical to those reported in New Zealand (Medsafe AEFI reports) and during
the pivotal clinical trial in non-pregnant peop e.(17)
The v-safe pregnancy register actively contacts participants who report they were pregnant at the
time of COVID-19 vaccination. Once enrol ed, they are contacted once per trimester, after delivery
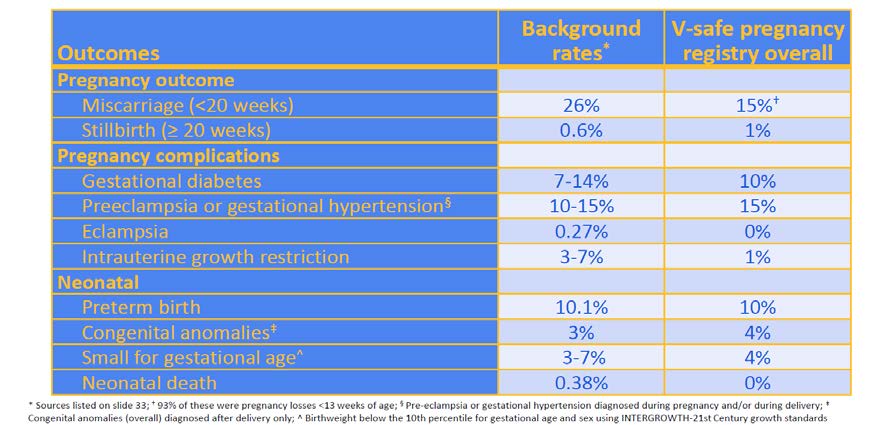
and when infant reaches 3 months of age to monitor for outcomes of interest. Preliminary data
showed that compared with known background rates, there was no increase in risk of adverse
pregnancy (miscarriage or still birth) or neonatal outcomes (preterm birth, small for gestational age,
congenital abnormalities or neonatal death) was seen in those who were vaccinated in the third
INFORMATION
trimester of pregnancy (see Figure 2).(15, 18) The data is limited for those who were vaccinated earlier
in pregnancy because the pregnancies are ongoing and not yet completed.
RELEASED UNDER THE
OFFICIAL

Document 5
IMAC – not for public distribution.
21 May 2021
Figure 2: V-safe pregnancy registry outcomes of interest in pregnant women vaccinated
against COVID-19, as of 18 February 2021 (CDC, presented by T Shimbukaro, 1 Mar 2021)
Reports to Vaccine Adverse Event Reporting System (VAERS) following vaccination in pregnant
ACT 1982
women (n=97) found no unexpected response, pregnancy or infant o tcomes with non-pregnancy
specific adverse events and miscarriage rates reflected that of th background rates. Vaccine safety
in pregnancy will continue to be monitored and bservational studies are ongoing.
One such observational study underway is called COVID-19 Vaccines International Pregnancy
Exposure Registry (C-VIPER) to specifically evaluate obstet ic, neonatal and infant outcomes among
women vaccinated pregnancy to prevent COVID-19 (NCT04705116).
Further data is anticipated, since as of 15 May 2021, over 270 million doses of COVID-19 vaccine
(including 144 million doses of Pfizer and 115 m l ion doses of Moderna mRNA vaccines) had been
administered in the US in total The UK is also monitoring COVID-19 vaccinations given in pregnancy
as part of the UK Vaccines in Pregnancy Surveillance Programme. The latest v-safe data (as of 17
May 2021) shows that almost 115,000 people using v-safe reported being pregnant when they
INFORMATION
received their COVID 19 vaccination (majority were given a mRNA vaccine) and nearly 5,000 were
enrol ed on the v-safe vaccine p egnancy registry.
RELEASED UNDER THE
Thrombosis with thrombocytopenia syndrome.
There have been r cent concerns around the risk of newly identified, very rare condition called
‘thrombosis with thrombocytopenia syndrome’ (TTS) occurring 4 to 20 days after the first dose of
adenovirus viral vector COVID-19 vaccines (AstraZeneca and Janssen vaccines). Prior to applying
preferred age restrictions, Australia saw six cases (five cases in women under 50 years of age and
one aged >80 years) of TTS after around a million doses and the UK has reported 209 cases after 22
OFFICIAL
million doses of Vaxzevria (AstraZeneca vaccine) – both in the ballpark of one per 100,000 vaccine
recipients.
Balancing the current risk from COVID-19 and the vaccine benefits, Australia prefers the mRNA
COVID-19 vaccine (Comirnaty) for those aged under 50 years, although, those who have had a first
dose of Vaxzevria may complete their immunisation with a second dose. People in the UK have a
higher risk from COVID-19, and so age-related risk-benefit has resulted in a preference is to give
mRNA vaccines in those younger than 30 years. For pregnant individuals of all ages, mRNA vaccine is
Document 5
IMAC – not for public distribution.
21 May 2021
preferred. No viral vector vaccines are currently approved for use in New Zealand to date, although
both AstraZeneca and Janssen vaccines are part of the advanced purchase agreements and Medsafe
continues to review the data for these. Note that this syndrome has not been associated with either
of the mRNA COVID-19 vaccines.(19)
Conclusions
COVID-19 is associated with a significantly increased risk of preterm delivery and maternal mortality.
Enhanced precautions to prevent SARS-CoV-2 infection in pregnancy are required. There is now a
large body of evidence from real-life surveillance data with no safety concerns emerging around
receiving COVID-19 vaccination in pregnancy with the mRNA vaccines. Decision-making needs to be
balanced with the risk of severe disease, particularly in those with underlying health conditions o
older age.
Most pregnant people in New Zealand have a very low risk of exposure to COVID-19. The current
advice is that those who are at low risk of exposure to COVID-19 may choose to delay vaccination
until after delivery. However, was there to be an outbreak of COVID-19 or if the borders open
further later in the year, unimmunised pregnant people will be vulnerable to infection, and this
change in risk as well as evolving data is likely to alter the current recommendations.
ACT 1982
Current international recommendations
(note see below for URLs if hyperlinks are not accessible)
Australia and New Zealand
Royal Australian and New Zealand Col ege of Obstetrics and Gynaecology (RNAZCOG)
[Updated 10 March 2021]
“Although the available data do not indicate any safety concern or harm to
pregnancy, there is insufficient evidence to recommend routine use of COVID-19 vaccines during
pregnancy. However, if a pregnant woman meets the definition of being particularly vulnerable, then
she should discuss the option of COVID-19 vacci ation with her obstetrician, GP and/or midwife. This
is based on the observation that people with certain underlying medical conditions are at very high
risk of experiencing se ious complic tions of COVID-19.
The most likely relevant groups of pregnant women include:
INFORMATION
• Significant pre-exi ting medical conditions e.g. diabetes
RELEASED UNDER THE
• Solid organ transplant recipients
• Those with chronic respiratory conditions including cystic fibrosis and severe asthma
• Those who have homozygous sickle cel disease
• Those receiving immunosuppression therapies sufficient to significantly increase risk of
infection
• Those receiving dialysis or with advanced chronic kidney disease
OFFICIAL
• Those with significant congenital or acquired heart disease
Pregnant workforce: RANZCOG recommends that, in settings of high community transmission, health
care workers with direct patient contact, and other workers in areas of significantly increased risk of
exposure to COVID-19, be al ocated to lower risk duties that have reduced risk of exposure to patients
with, or suspected to have, COVID-19 infection, working from home or leave of absence. RANZCOG
recognises that pregnant women are, appropriately, often anxious about their own health and
protective of their unborn baby. Where this is not possible to avoid exposure, pregnant workers who
Document 5
IMAC – not for public distribution.
21 May 2021
are in an at-risk work environment should be offered vaccination. All personnel should observe strict
hygiene protocols and have ful access to adequate Personal Protective Equipment (PPE).
General advice: Al medical advice should be patient-centred and take into account each individual’s
personal considerations and preferences. In the absence of evidence on the safety or efficacy of the
COVID-19 vaccines in pregnant women, the decision to receive vaccination rests solely with the
pregnant woman fol owing informed consultation with her midwife and/or doctor
.”
Canada
Society of Obstetricians and Gynaecologists of Canada
Consensus Statement [reaffirmed 4 May 2021]
:
1. Pregnant individuals should be offered vaccination at any time during pregnancy or wh le
breastfeeding if no contraindications exist.
2. The SOGC supports the use of al available COVID-19 vaccines approved in Canada in any
trimester of pregnancy and during breastfeeding in accordance with regional eligibility.
3. The decision to be vaccinated is based on the individual’s personal values as wel as an
understanding that the risk of infection and/or morbidity from COVID-19 outweighs the
theorized and undescribed risk of being vaccinated during pregnancy o while breastfeeding.
4.
ACT 1982
Individuals should not be precluded from vaccination based on pregnancy status or
breastfeeding.
5. Given that pregnant people are at increased ri k of morbi ity f om COVID-19 infection, al
pregnant persons should be eligible to receive a COVID-19 vaccination.
United Kingdom
Public Health England: [updated 30 April 2021]
The COVID-19 vaccines available in the UK have been shown to be effective and to have a good safety
profile. These vaccines do not contain live coronavirus and cannot infect a pregnant woman or her
unborn baby in the womb. There is no known risk with giving inactivated virus or bacterial vaccines or
toxoids during pregnancy o whilst breast feeding.
The Joint Committee on Vaccination and Immunisation (JCVI) has advised that pregnant women
INFORMATION
should be offered COVID-19 vaccines at the same time as people of the same age or risk group.
Evidence so far reviewed by the Medicines and Healthcare products Regulatory Agency (MHRA), the
RELEASED UNDER THE
UK regulatory agency responsible for licencing medicines including vaccines, has raised no specific
concerns for safety in pregnancy. Evidence on COVID-19 vaccines is being continuously reviewed by
the World Health Organization and the regulatory bodies in the UK, USA, Canada and Europe.
A leaflet ha been produced by PHE to guide women of child-bearing age, currently pregnant or
breastfeedi g. Pfizer and Moderna mRNA vaccines are the preferred vaccines for pregnant women
of any age who are receiving their first COVID-19 dose.
OFFICIAL
Public Health England has also established surveillance (UK Vaccine in Pregnancy Surveillance
programme) to monitoring inadvertent vaccination in pregnancy (to include COVID-19 vaccines,
varicella, shingles and MMR).
Royal College of Obstetricians and Gynaecologists
COVID-19 Vaccines, pregnancy and breastfeeding [Q&As updated 14 May 2021]:
Document 5
IMAC – not for public distribution.
21 May 2021
• The latest advice [16 Apr 21] from the Joint Committee on Vaccination and Immunisation
(JCVI) is that COVID-19 vaccines should be offered to pregnant women at the same time as
the rest of the population, based on their age and clinical risk group. Women should discuss
the benefits and risks of having the vaccine with their healthcare professional and reach a
joint decision based on individual circumstances.
• You should not stop breastfeeding in order to be vaccinated against COVID-19.
• Women trying to become pregnant do not need to avoid pregnancy after vaccination and
there is no evidence to suggest that COVID-19 vaccines wil affect fertility.
• Having a COVID-19 vaccine wil not remove the requirement for employers to carry out a risk
assessment for pregnant employees, which should fol ow the rules set out in this government
guidance.
A range of resources have been developed including an information sheet and decision aid for
pregnant individuals.
One Q&A that may be considered for the recommendations in New Zealand is:
Q. When in pregnancy can I have the vaccine?
The vaccine should work whatever the stage of pregnancy you are in. The JCVI advises that women do
not need a pregnancy test before vaccination, and that women planning a pregnancy do not need to
ACT 1982
delay pregnancy after vaccination.
However, as COVID-19 has more serious complications later pregnancy, some women may choose
to delay their vaccine until after the first 12 weeks (which are most important for the baby’s
development) and plan to have the first dose at any time rom 13 weeks onwards.
As pregnant women are more likely to be seriously unwel and have a higher risk of their baby being
born prematurely if they develop COVID-19 in their third trimester (after 28 weeks), women may wish
to have the vaccine before their third trimester
United States
CDC:
[updated 14 May 2021]
Pregnant people are more likely to get severely il with COVID-19 compared with non-pregnant
INFORMATION
people. If you are pregnant, you can receive a COVID-19 vaccine. Getting a COVID-19 vaccine during
pregnancy can p otect you from severe il ness from COVID-19. If you have questions about getting
RELEASED UNDER THE
vaccinated, a conversat on with your healthcare provider might help, but is not required for
vaccination.
American College of Obstetrics and Gynecology (ACOG):[updated 28 Apr 2021]
• ACOG recommends that pregnant individuals have access to COVID-19 vaccines.
• COVID-19 vaccines should be offered to lactating individuals similar to non-lactating
OFFICIAL
individuals.
• Individuals considering a COVID-19 vaccine should have access to available information about
the safety and efficacy of the vaccine, including information about data that are not available.
A conversation between the patient and their clinical team may assist with decisions
regarding the use of vaccines approved under EUA for the prevention of COVID-19 by
pregnant patients. Important considerations include:
o
the potential efficacy of the vaccine
Document 5
IMAC – not for public distribution.
21 May 2021
o
the risk and potential severity of maternal disease, including the effects of disease on
the fetus and newborn
o
the safety of the vaccine for the pregnant patient and the fetus.
• While a conversation with a clinician may be helpful, it should not be required prior to
vaccination, as this may cause unnecessary barriers to access.
Website URLs for hyperlinks
Australia and New Zealand
Medsafe COVID-19: Overview of vaccine reports: https://www.medsafe.govt.nz/COVID-19/vaccine-
report-overview.asp
Statement: https://ranzcog.edu.au/statements-guidelines/covid-19-statement/covid-19-vaccinatio -
information
Canada
Consensus statement:
https://www.sogc.org/common/Uploaded%20files/Latest%20News/SOGC Statement COVID-
19 Vaccination in Pregnancy.pdf
UK
ACT 1982
Public Health England Guidance: https://www.gov.uk/gove nment/publications/safety-of-covid-19-
vaccines-when-given-in-pregnancy/the-safety-of-c vid 19-vaccines-when-given-in-pregnancy
Patient leaflet:
https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment data/file
/965391/PHE COVID-19 vaccination guide on pr gnancy English v3.pdf
Q&A: https://www.rcog.org.uk/en/guidelines-research-services/coronavirus-covid-19-pregnancy-and-
womens-health/covid-19-vaccines-and-pregnan y/covid-19-vaccines-pregnancy-and-breastfeeding/
Decision aid: https://www rcog.org.uk/globalassets/documents/guidelines/2021-02-24-combined-
info-sheet-and-decision-aid pdf
INFORMATION
United States
https://www.cdc gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html
RELEASED UNDER THE
https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/vaccinating-
pregnant-and-lactating patients-against-covid-19
v-safe pregnancy registry: https://www.cdc.gov/coronavirus/2019-
ncov/vaccines/safety/vsafepregnancyregistry.html
OFFICIAL
References
1.
Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, et al. Clinical manifestations, risk
factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living
systematic review and meta-analysis. BMJ. 2020;370:m3320.
2.
Knight M, Bunch K, Vousden N, Morris E, Simpson N, Gale C, et al. Characteristics and
outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK:
national population based cohort study. BMJ. 2020;369:m2107.
Document 5
IMAC – not for public distribution.
21 May 2021
3.
Lokken E, Huebner E, Taylor GG, Hendrickson S, Vanderhoeven J, Kachikis A, et al. Disease
Severity, pregnancy outcomes and maternal deaths among pregnant patients with SARS-CoV-2
infection in Washington State. American Journal of Obstetrics and Gynecology. 2021.
4.
Mullins E, Hudak ML, Banerjee J, Getzlaff T, Townson J, Barnette K, et al. Pregnancy and
neonatal outcomes of COVID-19: co-reporting of common outcomes from PAN-COVID and AAP
SONPM registries. Ultrasound Obstet Gynecol. 2021.
5.
Walker KF, O'Donoghue K, Grace N, Dorling J, Comeau JL, Li W, et al. Maternal transmission
of SARS-COV-2 to the neonate, and possible routes for such transmission: a systematic review and
critical analysis. BJOG. 2020;127(11):1324-36.
6.
Liguoro I, Pilotto C, Bonanni M, Ferrari ME, Pusiol A, Nocerino A, et al. SARS-COV-2 infection
in children and newborns: a systematic review. European Journal of Pediatrics. 2020;179(7):1029-46.
7.
Bentov Y, Beharier O, Moav-Zafrir A, Kabessa M, Godin M, Greenfield CS, et al. Ovarian
follicular function is not altered by SARS-Cov-2 infection or BNT162b2 mRNA Covid-19 vaccination.
medRxiv. 2021:2021.04.09.21255195.
8.
Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology Nat
Rev Drug Discov. 2018;17(4):261-79.
9.
Abu-Raya B, Maertens K, Edwards KM, Omer SB, Englund JA, Flanagan KL, et al. Global
Perspectives on Immunization During Pregnancy and Priorities for Future Resea ch and
Development: An International Consensus Statement. Front Immunol. 2020;11:1282.
10.
Collier AY, McMahan K, Yu J, Tostanoski LH, Aguayo R, Ansel J, et al. mmunogenicity of
ACT 1982
COVID-19 mRNA Vaccines in Pregnant and Lactating Women. JAMA. 2021.
11.
Rottenstreich A, Zarbiv G, Oiknine-Djian E, Zigron R Wolf DG, Porat S. Efficient maternofetal
transplacental transfer of anti- SARS-CoV-2 spike antibodies after antenatal SARS-CoV-2 BNT162b2
mRNA vaccination. medRxiv. 2021 (Preprint):2021.03.11.21253352.
12.
Gray KJ, Bordt EA, Atyeo C, Deriso E, Akinwunmi B, Young N, et al. COVID-19 vaccine
response in pregnant and lactating women: a cohort study. American Journal of Obstetrics and
Gynecology. 2021.
13.
Drugs and Lactation Database (LactMed) COVID-19 vaccines. [Internet]Bethesda (MD):
National Library of Medicine (US); 2006- [19 Apr 2021. Available from:
https://www.ncbi.nlm.nih.gov/books/NBK565969/
14.
Perl SH, Uzan-Yulzari A, Klainer H, Asiskovich L, Youngster M, Rinott E, et al. SARS-CoV-2-
Specific Antibodies in Brea Milk After COVID-19 Vaccination of Breastfeeding Women. JAMA. 2021.
15.
Shimabukuro TT, Kim SY, Myers TR, Moro PL, Oduyebo T, Panagiotakopoulos L, et al.
Preliminary Findings of mRNA Covid 19 Vaccine Safety in Pregnant Persons. N Engl J Med. 2021.
INFORMATION
16.
Shimbabukuro T, CDC-COVID-19 Vaccine Task Force. COVID-19 vaccine safety update. March
2021. 2021 1 March 2021 Available from:
RELEASED UNDER THE
https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-02/28-03-01/05-covid-
Shimabukuro.pdf
17.
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy
of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603-15.
18.
Shimbabukuro T, CDC-COVID-19 Vaccine Task Force. COVID-19 vaccine safety update.
Februa y 2021. 2021 27 January 2021.Available from:
https://www.cdc.gov/vaccines/acip/meetings/slides-2021-1-27-21.html
OFFICIAL
19.
World Health Organization. Global Advisory Committee on Vaccine Safety (GACVS) review of
latest evidence of rare adverse blood coagulation events with AstraZeneca COVID-19 Vaccine
(Vaxzevria and Covishield) 2021 [updated 16 April 2021; cited 21 May 2021]. Available from:
https://www.who.int/news/item/16-04-2021-global-advisory-committee-on-vaccine-safety-(gacvs)-
review-of-latest-evidence-of-rare-adverse-blood-coagulation-events-with-astrazeneca-covid-19-
vaccine-(vaxzevria-and-covishield)
Document 5
IMAC – not for public distribution.
21 May 2021
ACT 1982
INFORMATION
RELEASED UNDER THE
OFFICIAL
Document 9
Draft statement on Long-term effects of Pfizer vaccine – for CVTAG review
Long-term safety of Pfizer vaccine
Medsafe independently reviewed the data on safety, quality and efficacy of the Pfizer vaccination
and approved it for use. This approval followed the same rigorous process that is used for all
previously approved medicines (including vaccines) currently licenced for use in New Zealand.
Based on the scientific understanding of how the Pfizer vaccine works, there have not been any
long-term effects predicted. However, Medsafe continues to monitor any side effects of the Pfizer
vaccine reported in New Zealand or from other countries where the Pfizer vaccine is in use. No long-
term effects have been suggested from this monitoring.
Additional information on the Pfizer vaccine’s long-term effectiveness, safety, and side effects is
expected from ongoing clinical trials, including trials in pregnant women and children. Participants
from the initial clinical trials are also being monitored for 2 years from their second dose of the
Pfizer vaccine with regular follow-up visits until 2023. Data from these trials is shared by
Pfizer/BioNTech with Medsafe and is being closely monitored.
ACT 1982
INFORMATION
RELEASED UNDER THE
OFFICIAL
Document 10
Strawman Statement
The new recommendation
We recommend that women/people who are pregnant are routinely offered COVID-19
vaccination at any stage of pregnancy. International surveil ance data of large numbers of
pregnant people indicate that there are no safety concerns with administering COVID vaccine
in any stage of pregnancy. Furthermore, the risk of an adverse outcome from Covid-19
infection during pregnancy is significantly higher compared to non-pregnant adults. There is
also evidence of antibody transfer in cord blood and breast milk which can offer protection
to infants through passive immunity. Pregnant women/people are encouraged to discuss
with their whānau and their LMC the decision to vaccinate or delay until after the baby is
born.
ACT 1982
INFORMATION
RELEASED UNDER THE
OFFICIAL


















