Comments on “Background notes relating to the nature and health significance and
persistence of trace of methamphetamine on indoor surfaces” by Dr Nick D Kim,
Senior Lecturer, School of Public Health, College of Health, Massey University
5 July 2016
Peter Cressey
ESR
The background note uses extant information to propose a level of surface contamination
with methamphetamine which could be viewed as the lowest plausible health-effects
concentration. While the general approach taken appears valid, some aspects of this
1982
analysis invite further elaboration.
Reference dose/health-based reference value
Act
To simplify terminology these values will be collectively referred to as health-based exposure
values (HBEVs). Two HBEVs are referred to in the background notes:
• A reference dose (RfD) of 0.3 µg/kg bw per day, derived by the Office of
Environmental Health Hazard Assessment (OEHHA), California Environmental
Protection Agency (CEPA) (Salocks, 2009).
• A reference value of 5-70 µg/kg bw per day, derived by staff from the Colorado
Department of Public Health and Environment (CDPHE) and the US Environmental
Protection Agency (USEPA) (Hammon and Griffin, 2007). While this value range is
referred to as a ‘reference value’ by the study authors, it is derived in a similar
Information
manner to a reference dose.
The RfD derived by OEHHA, CEPA is based on the study “Control of Weight Gain in
Pregnancy, Utilizing Methamphetamine” (Chapman, 1961). This was a double-blind placebo-
controlled trial of the efficacy of methamphetamine for controlling weight gain during
pregnancy. Four dose groups were included, receiving methamphetamine doses of 0.0,
Official
0.08, 0.15 and 0.17 mg/kg bw per day. At the conclusion of the study, the dose levels had
changed slightly due to weight loss/gain by some participants. Weight gain was significantly
different in all treatment groups compared to the control group. While some side-effects were
the
noted the overall prevalence did not appear to be dose-related, with similar total side-effects
in the control and high-dose groups.
Babies born to mothers who had lost weight “appeared normal and healthy”. All
electrocardiogram and laboratory results for all patients evaluated were within normal
under
ranges.
OEHHA, CEPA judged maternal weight loss to be an adverse effect, giving a Lowest
Observed Adverse Effect Level (LOAEL) of 0.08 mg/kg bw per day (Salocks, 2009). Three
components of uncertainty were applied in deriving a RfD:
• Extrapolation from the LOAEL to a No Observed Adverse Effects Level (NOAEL)
(x10). Uncertainty factors for LOAEL to NOAEL extrapolation are usually in the range
3-10, with higher factors used if the effect seen at the LOAEL in considered serious.
• Inter-individual uncertainty factor (x10)
Released
• (In)completeness of database (x3)
These factors result in an overall uncertainty factor of 300 and a RfD of 0.00026 mg/kg bw
per day (rounded to 0.0003 mg/kg bw per day or 0.3 µg/kg bw per day).
In the context of the Chapman study, there must be some questions as to whether the
weight loss seen represents a true adverse effect. Similarly, it must be questioned whether
this effect is sufficiently serious to warrant application of a 10-fold uncertainty factor for
extrapolation from LOAEL to NOAEL. However, derivation of a RfD based on the Chapman
study has two advantages; it was carried out in humans and requires no interspecies
extrapolation, and doses were administered by the oral route.
The reference value derived by CDPHE/USEPA was derived using benchmark doses
(BMDL10 – the lower 95th percentile confidence limit for a dose giving a 10% change in
response compared to baseline) derived from animal reproductive and developmental
toxicity studies (Hammon and Griffin, 2007). BMDL10 values were in the range 1.5 to 20
mg/kg bw per day, with the lowest BMDL
1982
10 relating to decreased foetal weight. A total
uncertainty factor of 300 was applied to the BMDL10 values, made up of components for
inter-species extrapolation (x10), inter-individual extrapolation (x10) and database
deficiencies (x3). This resulted in a range of reference values from 0.005 to 0.066 mg/kg
Act
bw/day, rounded to 5-70 µg/kg bw per day.
It should be noted that all animal studies used non-oral routes of dose administration
(intravenous, intraperitoneal or subcutaneous) and are not immediately comparable to the
routes of exposure to methamphetamine that would occur in a contaminated house.
In the background notes, Dr Kim chose to use the latter reference values (CDPHE/USEPA)
as a reference point for human methamphetamine exposure, rather than the human-derived
OEHHA RfD. The rationale for this decision was that “an RfD provides a level at which long-
term exposure is without appreciable risk, a health-based reference value provides the
Information
lowest level at which the first onset of the most sensitive possible health effect may begin to
occur”. I believe that this overstates the difference between these two approaches. The
CDPHE/USEPA study bases the derived HBEV on benchmark doses, from animal studies.
There has been a general move to the use of benchmark doses, rather than NOAELs,
because the benchmark dose can be determined with some confidence by interpolation of
the dose response curve, while the NOAEL is the highest dose at which no adverse effect is
Official
seen and is not necessarily a point on the dose-response curve (see attached illustrative
Figure 1). While uncertainty factors are applied to a NOEAL to arrive at a HBEV that is
without appreciable risk (but not ‘without any risk’), benchmark doses are most often
the
compared to human estimated exposures, with the resulting margin of exposure (MOE)
being a measure of the level of concern associated with the human exposure. The study of
Hammon and Griffin essentially predefines a MOE of 300. MOEs of this magnitude, for
threshold effects (non-carcinogenic) would usually be considered to represent a low level of
toxicological concern. I believe that ‘without appreciable risk’ and ‘a low level of toxicological
under
concern’ cannot be viewed as distinctly different expressions of the level of risk.
In my opinion, Dr Kim’s choice of the higher HBEV requires a more substantial rationale than
has currently been provided. In the absence of a clear reason to choose one HBEV over
another, a conservative approach to risk assessment would suggest that the lower HBEV
should be used as the reference point for assessing risks.
Given the approximately 17-fold difference between the two HBEVs, this decision had a
major impact on Dr Kim’s conclusions. It should also be noted that the toxicological endpoint
Released
associated with the reference value used by Dr Kim is decreased foetal weight, which is of
questionable relevance to the most sensitive exposed individuals in a methamphetamine-
contaminated property (infants).
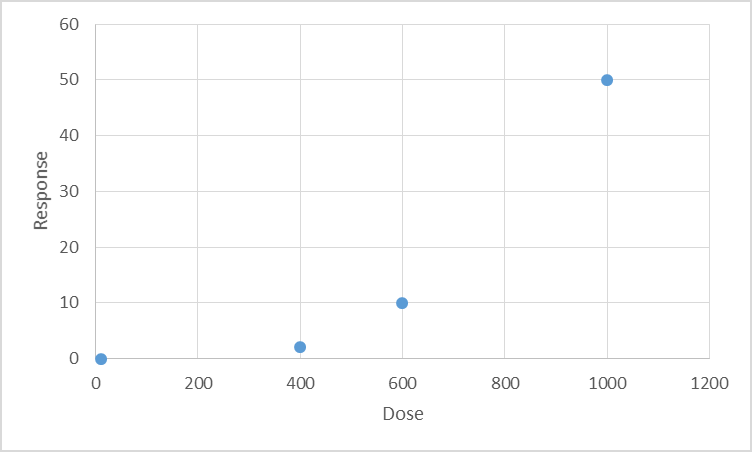 Figure 1. Il ustrative figure showing various toxicological points of departure in relation to a hypothetical dose-response
curve
Figure 1. Il ustrative figure showing various toxicological points of departure in relation to a hypothetical dose-response
curve
‘True’ NOAEL could be anywhere
in this range
LOAEL
NOAEL
BMD
1982
Act
Human exposure to methamphetamine due to surface contamination
Information
Dr Kim uses the exposure assessment carried out by Hammon and Griffin (2007), to
determine human exposure resulting from different levels of surface methamphetamine
contamination, as the basis for his conclusions. The approach taken in the study of Hammon
and Griffin appears to be satisfactory and determines that exposure will be predominantly by
oral exposure resulting from surface to hand to mouth transmission. Dermal absorption was
assessed to contribute a lesser amount to methamphetamine exposure. Hammon and Griffin
Official
calculated exposure as an ‘internal dose’ assuming 100% absorption of oral doses and 10%
absorption of dermal doses. The highest estimated exposures were for infants and ranged
from 0.019 µg/kg bw per day at a surface contamination of 0.05 µg/100 cm2 to 0.19 µg/kg bw
the
per day at a surface contamination of 0.5 µg/100 cm2 (the current New Zealand guideline
level).
The exposure model used by Hammon and Griffin has a linear relationship between surface
methamphetamine contamination and human exposure. Dr Kim used the linearity of this
relationship to determine that exposure equivalent to the lowest Hammon and Griffin
under
reference value (5 µg/kg bw per day) would result from a surface methamphetamine
contamination of 12.5 µg/100 cm2. It should be noted that, by the same reasoning, the
OEHHA RfD exposure level would equate to a surface methamphetamine contamination of
0.8 µg/100 cm2. While the RfD was derived from an external (administered) dose and the
exposure estimates of Hammon and Griffin are internal doses, the assumption of 100% oral
absorption of methamphetamine used by Hammon and Griffin means there is no conflict
between the bases used for the exposure dose and RfD.
Released
Use of the term ‘contaminated’ Dr Kim argues that surface methamphetamine concentrations of less than 12 µg/100 cm2
should not be referred to as ‘contamination’, as this level is unlikely to be hazardous. Dr Kim
contends that ‘contaminated’ should only apply in situation where the substance is present at
concentrations sufficient to result in harm.
In my opinion, methamphetamine can reasonably be considered to be a contaminant, rather
than a normal component, of the domestic environment. Normal dictionary definitions of
contamination refer to the presence of something undesirable, rather than its presence at a
level high enough to cause harm. Methamphetamine’s presence, at any concentration, is
likely to be unwanted and undesirable. Therefore, if methamphetamine is a contaminant,
then any measurable concentration of methamphetamine in a house could lead to the house
being described as contaminated with methamphetamine.
Indeed, in some cases a normal component of a medium may still be referred to as a
contaminant. For example, the heavy metal mercury may be naturally present at appreciable
concentrations in fish. Mercury is conventionally referred to as a contaminant in this context,
even when present at low concentrations.
1982
References
Act
Chapman JD. (1961). Control of weight gain in pregnancy, utilizing methamphetamine.
Journal of the American Osteopathic Association; 60: 993-997.
Hammon TL, Griffin S. (2007). Support for selection of a methamphetamine cleanup
standard in Colorado. Regulatory Toxicology and Pharmacology; 48(1): 102-114.
Salocks C. (2009). Development of a Reference Dose (RfD) for Methamphetamine
.
California Environmental Protection Agency. Office of Environmental Health Hazard
Assessment. Integrated Risk Assessment Branch.
Information
Official
the
under
Released
